The ‘Laboratory for Cell Mimicry’ aims to employ bottom up assemble synthetic entities to assembled live-like units that could integrate with their natural counter parts. We work across the length scale, from synthesizing organic compounds as enzyme mimics over the assembly of catalytic nanoparticles as artificial organelles to the engineering of artificial cells and their integration with mammalian cells into bionic tissue.
Artificial organelles are nanosized reactors with intracellular activity, often encapsulated catalysis. In contrast to traditional pharmaceutical, artificial organelles are envisioned to provide a sustained solution for a chronic condition.
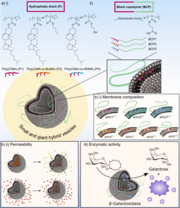
Hybrid vesicles (HVs), formed by combining phospholipids and amphiphilic block copolymers (BCPs), offer a contemporary alternative to liposomes and polymersomes. By altering the chemical composition of the hydrophobic block in the BCPs, specifically using statistical copolymers of cholesteryl methacrylate and either butyl methacrylate (BuMA) or 2-hydroxyethyl methacrylate (HEMA), variations in HV membrane properties were achieved. Membranes with HEMA-containing BCPs exhibited higher permeability, while those with BuMA provided an optimal environment for efficient association with β-galactosidase, demonstrating the significance of the hydrophobic block in controlling HV morphologies and properties.
Artificial cells are larger micron-sized assemblies that aim at structurally and functionally support mammalian cells and tissue.

Artificial cells, designed to support mammalian cells, feature minimal liver-like functions using alginate and metalloporphyrins mimicking CYP1A2 enzyme activity. Integrated into 3D bioprinted structures with HepG2 cells, these artificial cells enhance dealkylation activity, monitored by resorufin conversion. The composite ink, combining artificial cells and HepG2 aggregates, sustains CYP1A2-like activity for at least 35 days, demonstrating a promising step in bottom-up synthetic biology for tissue engineering.

Cellular communication is vital for the coordinated adaptation of multicellular tissues to environmental changes. In the context of integrated semi-synthetic systems with artificial cells (ACs) and mammalian cells, this study demonstrates that ACs can eavesdrop on HepG2 cells by monitoring the activity of cytochrome P450 1A2 (CYP1A2). The ACs utilize d-cysteine as a signaling molecule, triggering a cascade of enzymatic reactions in HepG2 cells, leading to the production of d-luciferin and subsequent luminescence in the ACs, providing a method to assess the level of hepatic CYP1A2 function.
Self-propelled particles attract a great deal of attention due to the auspicious range of application nanobots can be used for. In a biomedical context, self-propelled swimmers hold promise to autonomously navigate to a desired location in an attempt to counteract cell/tissue defects either by releasing drugs or performing surgical tasks. Self-propelled particles attract a great deal of attention due to the auspicious range of application nanobots can be used for. In a biomedical context, self-propelled swimmers hold promise to autonomously navigate to a desired location in an attempt to counteract cell/tissue defects either by releasing drugs or performing surgical tasks.
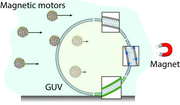
Self-propelled nano/micromotors, crucial for applications in nanomedicine like imaging and drug delivery, were investigated in this study. Magnetically propelled motors of varying sizes and surface chemistries were tested for their ability to traverse lipid membranes in giant unilamellar vesicles (GUVs). Surprisingly, size alone did not dictate membrane-crossing ability; PEGylated motors of 0.5 μm size showed limited interaction compared to their positively charged counterparts of the same size, and membranes with saturated lipids facilitated crossing irrespective of motor size. The study offers valuable insights for future motor designs by considering both ensemble and individual motor behavior in interactions with biological barriers.

A novel in vitro epidermis model using floating paper chips as a scaffold for primary human keratinocytes demonstrates the formation of all four epidermal layers, including the cornified layer, tight junction formation, and organelle alterations during differentiation. This model is employed to assess keratinocyte migration, and magnetic micromotors, assembled on the paper chips, are confirmed to aid cell migration under a static magnetic field, presenting a promising approach for studying skin disease pathologies and treatment evaluations.