Caught soap-handed: Understanding how soap molecules help proteins get in and out of shape
Controlling protein structure is crucial in the production of detergents and cosmetics. Up to now we have not had a clear understanding of how soap molecules and proteins work together to change protein structure. Now AU researchers have succeeded in creating a detailed picture of both unfolding and refolding of a protein by soap molecules on the millisecond timescale.
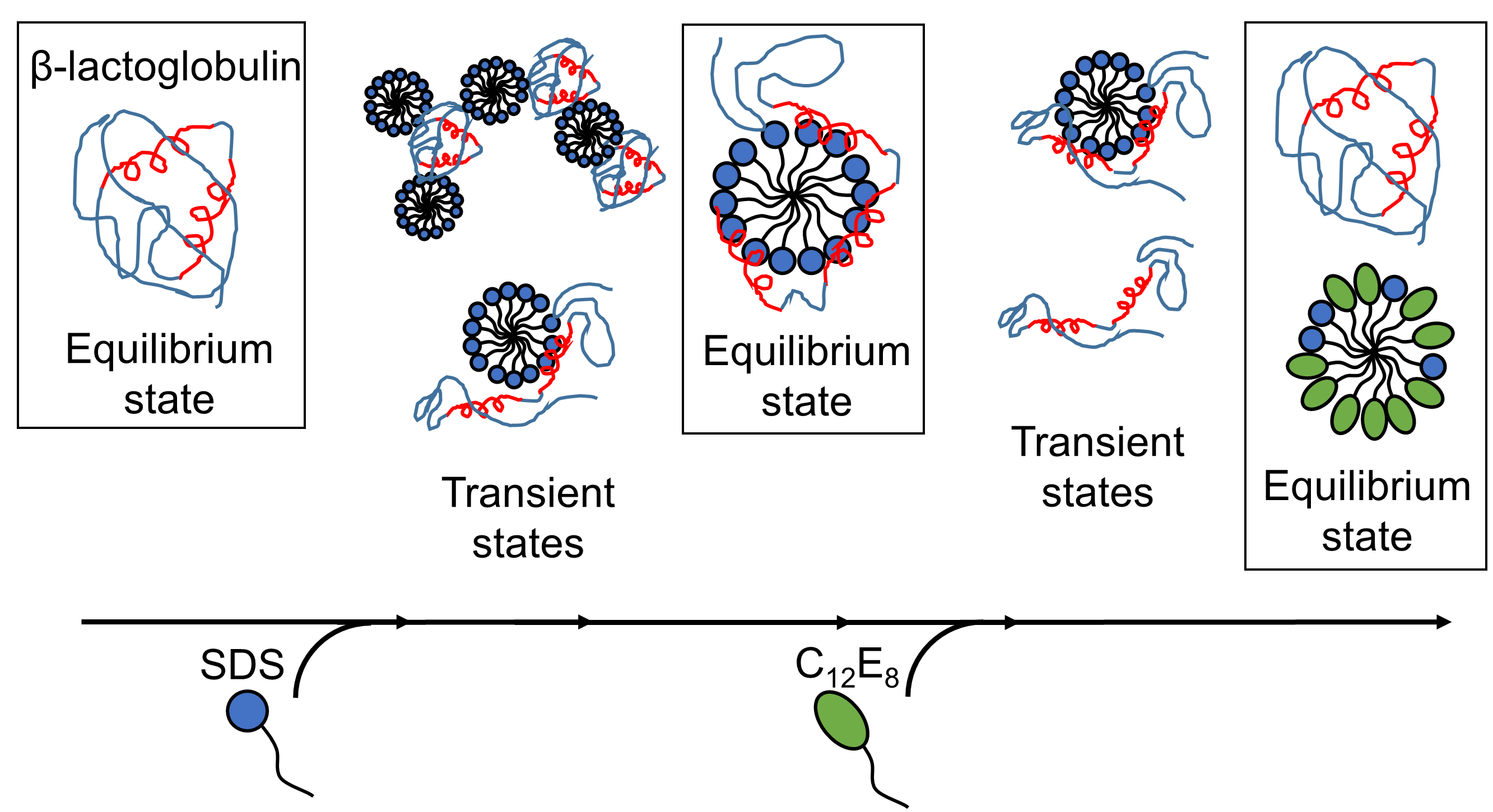

Understanding the interactions between proteins and soap molecules (surfactants) has long been important for the industry, particularly within detergents and cosmetics. The anionic surfactant sodium dodecyl sulfate (SDS) is known to unfold globular proteins, while the nonionic surfactant octaethylene glycol monododecyl ether (C12E8) does the opposite, i.e. it helps proteins fold into shape again.
For washing powders to work efficiently, it is important that the surfactants do not change the structure of proteins (enzymes), as any change in enzyme structure kills their ability to break down stains and remove dirt. Most washing powders contain mixtures of surfactants which allows the enzymes to remain active. Also, some biotechnologies exploit surfactants in combination with proteins.
Membrane proteins usually sit in the cell membrane. In order to extract them from this environment for different studies, they have to be solubilized by surfactant. The surfactant has to be ‘gentle’ and only cover the membrane-inserted part of protein so that their structure is preserved. In contrast, when characterizing the molecular weight of proteins in the lab, a standard technique is to unfold them by the aggressive negatively charged surfactant, SDS, and monitor how they migrate in a polymer gel in an electric field. This technique only works if the surfactant completely unfolds the proteins and destroys their structure.
There is still debate about which type of interactions between the protein and the surfactant is most important. Is it the electrostatic interactions between the charges of the surfactant and the protein, or is it simply the properties of the interface of the aggregates (micelles) that the surfactants form in water, which are responsible for the unfolding of the protein?
While unfolding has been studied in detail at the protein level, a complete picture of the interaction between protein and surfactant is lacking in these processes. This lack of knowledge is addressed in the current work using the globular protein β-lactoglobulin (bLG) as a model protein.
The right combination of experimental techniques
Deeper insight into unfolding and refolding of proteins was obtained, as the various steps of interactions between surfactant and proteins were mapped out as a function of time. Firstly, the model protein, bLG, was mixed with the anionic surfactant SDS while following the time evolution of the formation of complexes between protein and surfactant molecules on the millisecond-minute time scale. By this the researchers have determined the structure of the evolving complexes. Subsequently they mapped the time course of the refolding process when non-charged surfactant (C12E8) was added to a sample containing complexes of SDS and protein.
In order to observe how the protein rearranges during the unfolding and refolding process induced by surfactants, complementary spectroscopic techniques, Circular Dichroism and tryptophan fluorescence, were used in combination with time-resolved Small-angle X-ray scattering (SAXS).
Circular Dichroism and tryptophan fluorescence monitor changes in the structure of bLG, while changes in the overall shape of the protein–surfactant complexes were followed by synchrotron SAXS. This combination of techniques has not been used before to study these processes.
Complex processes lasting milliseconds to minutes
The unfolding of the protein by SDS was a homogeneous process, where all protein molecules follow the same unfolding route. The SDS complexes (micelles) attack the protein molecules head-on and then gradually unfold the protein so that it forms a shell around the SDS micelle. Refolding kicks off when C12E8 micelles “suck out” SDS from the protein-SDS complex to form mixed SDS-C12E8 micelles. However, the actual refolding process seems to follow several routes, since multiple structures were found to form in parallel, namely protein-surfactant complexes (probably containing both SDS and C12E8), mixed micelles of SDS and C12E8, “naked” proteins unfolded like long polymeric chains, and properly folded proteins. The experiment allowed the interconversion between these species to be followed, so that it could be determined which of the processes are fast and which ones are slow. The folded protein could form both from the naked unfolded proteins (quickly) and from protein-surfactant complexes (more slowly). Thus, the best way in which surfactants can help a protein to fold is to basically get out of the way and let the protein find its own way back to the folded state.
The results have provided deeper insight into the structural changes occurring at the protein–surfactant level. They reveal that surfactant-mediated unfolding and refolding of proteins are complex processes of rearrangements occurring on time scales from below milliseconds to minutes and involve intimate collaboration between surfactant complexes and proteins.
READ ALSO: Empty spaces, how do they make a protein unstable?
More information
The work is financially supported by the Independent Research Fund Denmark.
The research was carried out by researchers from Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, and Department of Molecular Biology and Genetics at Aarhus University (AU), in collaboration with researchers from ESRF - The European Synchrotron in Grenoble (France). Professor Jan Skov Pedersen (iNANO and Department of Chemistry, AU) and Professor Daniel E. Otzen (iNANO and Department of Molecular Biology and Genetics, AU) were in charge of the research team.
Read more about the results in Chemical Science (Open Access):
Contact
Professor Jan Skov Pedersen
Email: jsp@chem.au.dk
Tel: +4587155921
Professor Daniel Otzen
Email: dao@inano.au.dk
Tel: +4587156741
