DsbB
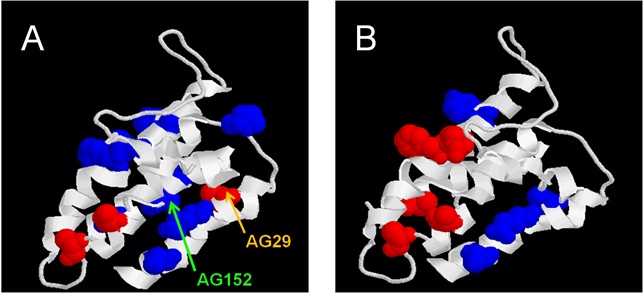
I have adopted this 4-transmembrane helix as a small model for membrane protein folding. The protein can be refolded from the SDS-denatured state into detergents and lipids (1). Extending previous work by others using mixed micelles between dodecyl maltoside and sodium dodecyl sulphate to measure thermodynamic stability of membrane proteins, I have developed a kinetic approach to determine both stability and the folding and unfolding mechanism of a membrane protein, the first such study (1). This showed that the protein minimally follows a 3-state folding pathway. We have recently extended this to a thermodynamic study over the temperature range 15-45oC, which in combination with equilibrium denaturation experiments highlights an unusual decrease in heat capacity upon unfolding, probably reflecting the specific contributions from the amphiphilic environment (2). More recently, my extensive study of a series of side-chain mutants of DsbB identified a polarized folding pathway, in which one part of the protein is significantly more structured than the other in the intermediate state and transition state of unfolding (3). In the figure below, residues which are relatively folded are shown in red and residues relatively unfolded (closer to the unfolded state than the folded state) are in blue. (A) shows the intermediate and (B) the transition state between the intermediate and the native state. The unstructured part of DsbB in the transition state and intermediate states (the upper part of the protein in the figure) is close to the periplasmic space. This suggests that the processes driving folding emerge from the part that is most deeply embedded in the lipid bilayer and for whom it may be easiest to start to develop tertiary structure due to their proximity in the membrane environment. In contrast, the exterior loops may be subsequently brought together by the assembly of the transmembrane helices which then act as an organizing scaffold.
The autotransporter AIDA
The bacterial outer membrane protein AIDA (Adhesin Involved in Diffuse Adherence) is an autotransporter, and as such it inserts spontaneously in the outer membrane via the 336-residue b-domain, after which the 900-residue a-domain is translocated across the outer membrane to be displayed on the surface. We have recently reported a detailed study of the folding of the isolated b-domain (13), which shows that it can only be refolded when immobilized on a column; attempts to refold it in solution leads to misfolded states which display cooperative unfolding but remain proteolytically sensitive and lack the band-shift upon heating which is characteristic of folded outer membrane proteins. We have been unable to identify any periplasmic factors, including purified periplasmic chaperones, which might help folding of this domain, suggesting that an interaction mediated by the a-domain might facilitate folding (in prep. and (14)). The folded state of AIDA can be unfolded in the presence of SDS only at high temperatures, and there is a linear relationship between the SDS mole fraction and the melting temperature (13). A very detailed study of thermal scans in the presence of various detergents has shown that it is the micellar, rather than bulk, detergent composition that is the appropriate parameter to use in this analysis (15).
Complementary to this work, we have also studied the interactions between different detergents and lipids by spectroscopic and surface tension techniques. This sheds more light on the characteristics of mixed micelle systems which is central to our analysis of membrane protein behaviour in amphiphiles (16, 17).
The outer membrane protein OmpA
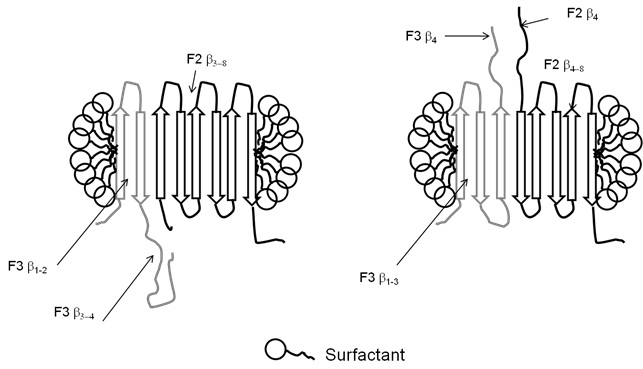
OmpA is one of the most well-studied outer membrane proteins (OMPs) and is also the most abundant OMP in E. coli. It consists of an N-terminal 16 β-strand β-barrel membrane-bound domain as a well as a periplasmic C-terminal domain. Whereas ?-helical membrane proteins like bacteriorhodopsin are able to fold by association of individual helices, Jörg Kleinschmidt and Lukas Tamm as well as others have shown OmpA to fold in a much more concerted mechanism. However, Ralf Koebnik showed that OmpA can actually "reconstruct" itself and form functional membrane-bound domains if different parts of the protein are expressed separately (in trans) in vivo. We have developed ways to follow this process in vitro using the membrane-bound domain of OmpA with a protease-cleavable linker and have shown that the rate of association of the fragments depends dramatically on where the protein is cleaved (4). For overlapping fragments, there is clearly competition between which fragment's parts get integrated, but there is a tendency for the big fragment to win out (see figure below) (4).