Publication in ACS Sustainable Chem. Eng. in collaboration with Matthias Beller!
Now online!! The work on deaminative hydrogenation of amides under mild conditions! Congrats to Laurynas for a successful internship at Matthias Beller's group in Rostock.
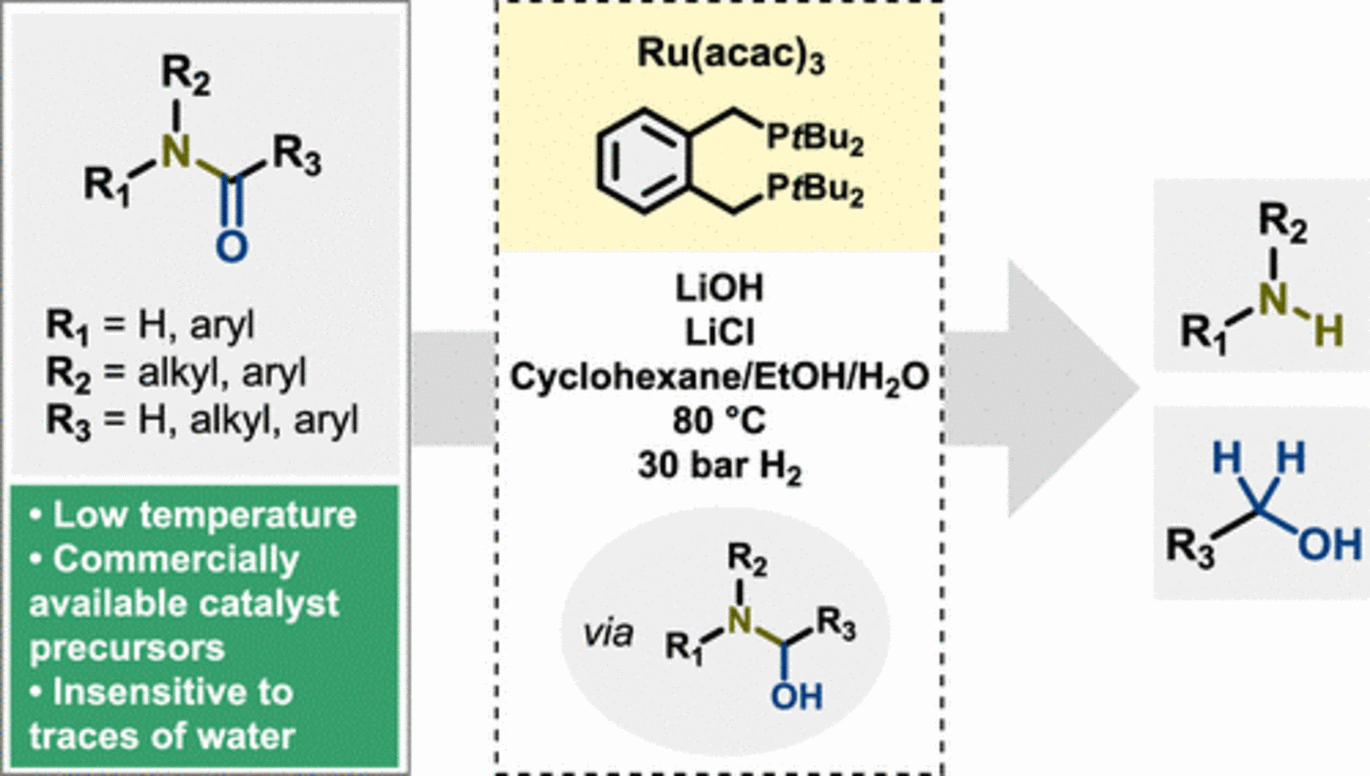
Abstract: Amide bond reduction is a versatile transformation offering access to various alcohols and amines that could be used as valuable precursors in the chemical and pharmaceutical industries, e.g., for manufacturing plastics, textiles, dyes, agrochemicals, etc. Over the last two decades, catalytic amide hydrogenation employing homogeneous catalysis has gained more attention due to the atom efficiency and low environmental impact of this transformation. Owing to the inherent strength of amide bonds, amide hydrogenation procedures often involve high temperatures and pressures, which is why efforts are being channeled to finding protocols with lower-energy input. Here, we report a mild amide hydrogenation method involving commercially available precursors Ru(acac)3 and 1,2-bis(di-tert-butylphosphinomethyl)benzene (L4), which under basic conditions, at 80 °C and under 30 bar of H2, can selectively hydrogenate a series of 2°-benzamides to anilines and alcohols with yields of 36–98% and 29–92%, respectively. Additionally, 1°- and 3°-amides proved to be appropriate substrates; however, low to moderate yields were obtained. The catalyst is believed to operate via an inner-sphere mechanism with a hemiaminal being the likely intermediate during the hydrogenation process.