Publication in ChemElectroChem
Check out our newest publication in collaboration with Kim Daasbjerg's group and Morten Ahlquist's group!
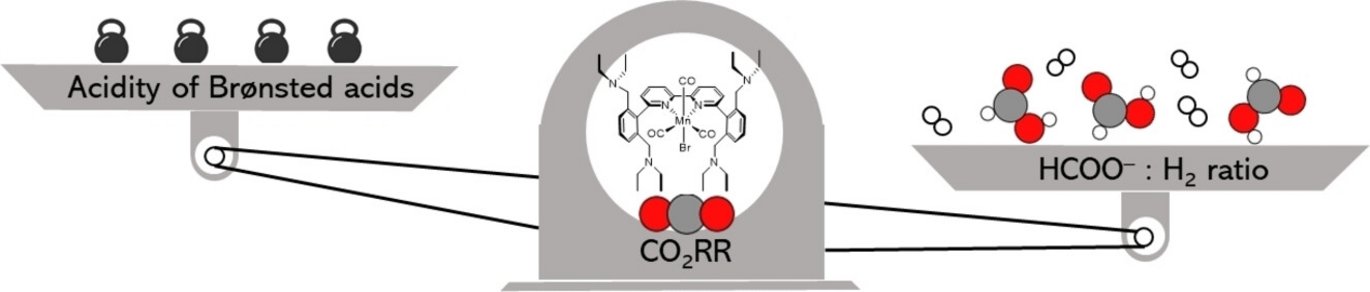
Abstract: The increasing concentration of CO2 in the atmosphere and its impact on the climate are matters of significant concern. Extensive research is being conducted on molecular catalysts to electrochemically reduce CO2 into valuable products to disrupt the unidirectional carbon flow. This study compares two manganese bipyridine catalysts, tailored with four or two benzylic diethylamine groups in the secondary coordination sphere. Either of these amine-bearing scaffolds positioned close to the Mn center serves as effective proton relays to facilitate the formation of the corresponding Mn hydride intermediate. Alongside competitive H2 evolution, the reaction of this crucial intermediate with CO2 leads to formate. Our findings underscore the pronounced influence of external Brønsted acids on product selectivity. Notably, when employing the catalyst bearing four amine groups, the HCOO−/H2 ratio varies from 81 : 3 with 1.0 M iPrOH to 16 : 64 with 1.0 M PhOH, while the Mn complex adorned with two amine pendant groups consistently favors HCOO−, irrespective of the utilized proton sources. Infrared spectroelectrochemistry and density-functional theory calculations unveil distinct disparities in the reactivity of the Mn hydrides toward CO2 due to the change of ligand bulkiness in the two cases. This work substantiates the importance of modulating spatial accessibility while modifying the second sphere encompassing molecular catalysts.
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/celc.202300553