Has arginine been overlooked as a target for bioconjugation on antibodies?
A team of researchers from iNANO and Department of Chemistry have synthesized a molecule containing diketopinic acid and an azide handle which allows for chemo-selective bioconjugation to arginine. This novel conjugation method offers precise and stable bioconjugation to proteins, enhancing the potential for various biomedical applications.
Has arginine been overlooked as a target for bioconjugation on antibodies?
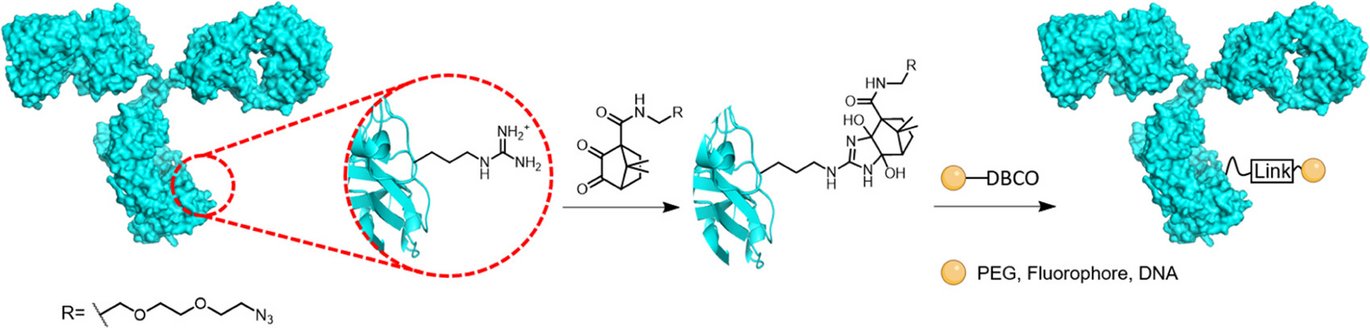
Scientists from the group of Professor Kurt V. Gothelf have developed a novel compound that could advance the use of antibodies in diagnostics and targeted therapies. The new compound, based on diketopinic acid (DKPA), offers a precise and reversible method to label antibodies, allowing researchers to better control antibody modifications without compromising function.
Current bioconjugation methods—techniques used to attach markers to proteins for medical applications—often struggle with precision, as they frequently bind to more common amino acids found all over antibody surfaces. This DKPA-based compound, however, selectively binds to a few arginine residues, which are less common and easier to target for a more uniform result. This innovation could lead to more reliable and consistent antibody-based drugs and diagnostic tools.
Notably, the compound’s binding is reversible, allowing researchers to label and unlabel antibodies without affecting stability. Through testing, the researchers showed that the labeled antibodies retain their ability to target cancer cells, a critical feature for therapies like those targeting breast cancer. Additionally, the compound performed well across different antibody types and maintained stability over days of testing. This stability, combined with high specificity, has promising implications for precise drug delivery, imaging, and the future of personalized medicine.
“This new compound could vastly improve the accuracy and adaptability of antibody-based technologies,” noted Professor Kurt V. Gothelf. With potential applications ranging from cancer treatment to precise diagnostic tools, the DKPA-based reagent represents a significant advancement in biomedicine.
About the research
Study type:
Experimental chemistry
External funding:
We are grateful for financial support for this study by the Novo Nordic Foundation Challenge Center for Multifunctional Biomolecular Drug Design (Grant No. NNF17OC0028070). We are also grateful for the Agilent liquid chromatography time-of-flight mass spectrometer 6230 granted to us by the Carlsberg Foundation (Grant No. CF21-0579).
Conflicts of interest:
The authors declare no competing financial interest.
Link to the scientific article:
https://pubs.acs.org/doi/full/10.1021/acs.bioconjchem.4c00317
Mathias B. Bertelsen, Emily Tsang, Johan Palmfeldt, Celine H. Kristoffersen, Marija Nisavic and Kurt V. Gothelf
Contact information:
Professor Kurt Vesterager Gothelf
Aarhus University
Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry
Email: kvg@chem.au.dk
