Nanostructures with improved stability for the development of more effective cancer nanomedicine
Most drugs used today have only one mechanism of action and it is both difficult and expensive to manufacture drugs with multiple functions. Researchers at the NNF-funded research center, CEMBID, at Aarhus University have recently found a way to create more stable nanostructures that can assemble biomolecules with different functions, which in combination e.g., can provide more effective cancer medicine.

For millennia, DNA has played a central role in storing each cell's genetic information and consists of strands with a specific sequence of four different building blocks. These DNA strands are copied by the cell at each cell division in an extremely well-orchestrated way, but amazingly, this sophisticated machinery is governed by very simple rules.
In recent years, it has been found to utilize these simple rules not only in the context of genetic engineering, but also to construct useful DNA nanostructures by designing DNA strands. These DNA nanostructures have been shown to have a number of useful biomedical functions such as to be able to transport cancer drugs to exact places in the body where they are needed. This can increase the effect of the medication as well as provide fewer side effects compared to conventional cancer treatment.
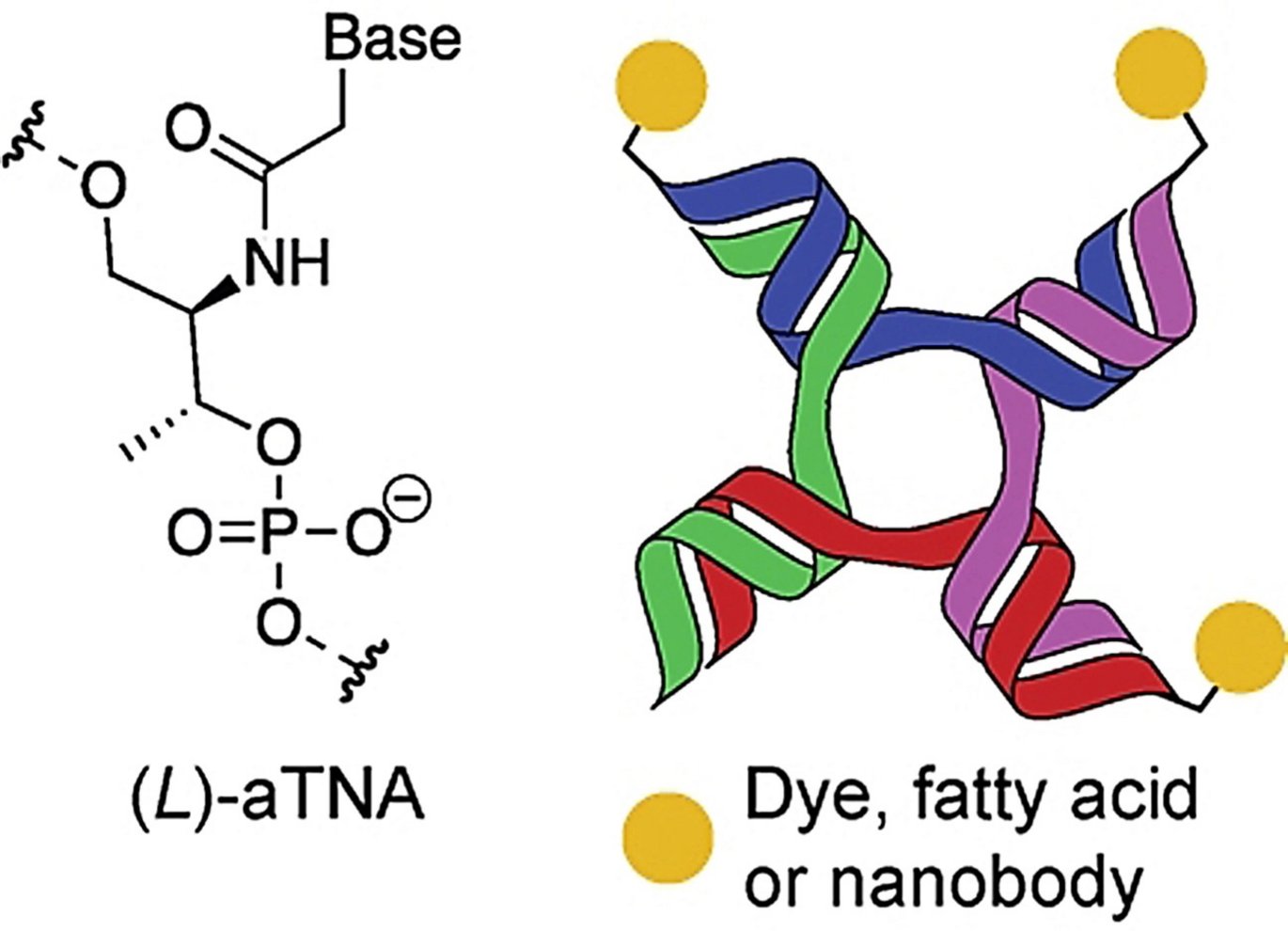
DNA nanostructures are also increasingly used as a tool to bind and assemble biomolecules, into multifunctional structures. One of these DNA nanostructures used forms a branched structure with four ends (see illustration), called 4-way junctions (4WJ), which are also found naturally.
With specially designed versions of these 4WJ structures, for example, Harvard Medical School in Boston has managed to bind and collect various antibodies, which in combination ensured that T cells attacked aggressive cancer cells more intensively and thus killed tumors.
Enhanced DNA nanostructures with artificial building blocks
Researchers who are part of the Center for Multifunctional Biomolecular Drug Design (CEMBID) at Aarhus University are also working on finding new ways to link different drugs to achieve more and more effective mechanisms of action. The research group, led by Professor Kurt Gothelf, has just published an article in the acclaimed journal Angewandte Chemie Int. Ed. with results involving above mentioned 4WJ structures, but in an improved version. The work was performed in collaboration with the groups of Jørgen Kjems and Ken Howard who are also part of CEMBID.
Admittedly, these DNA nanostructures (4WJ) are smart, but there is the disadvantage of DNA structures that DNA is de facto a biodegradable polymer. This means that the structures are broken down faster in the blood than desired. In addition, the structures can be so large that they themselves activate the immune system. In order for the structures to be used for diagnostics or in medicine, it is crucial that the structures are very stable, non-toxic and do not themselves trigger an immune reaction in the patient.
Anders Märcher, a postdoc in Kurt Gothelf's research group and part of CEMBID, has, together with his research colleagues, now found a way to increase the stability of these nanostructures. They have achieved this by using small chains, called oligonucleotides, of artificial and modified building blocks to form the nanostructure. The artificial oligonucleotides, Märcher et al. use is called acyclic L-threoninol nucleic acid (aTNA) and work in the same way and just as well as the natural building blocks of DNA. Here, the sugar molecule (deoxyribose) in the natural building blocks is replaced with an artificial sugar molecule (acyclic L-threoninol), which strengthens the overall structure.
The positive results showed that 4WJ structures with the artificial building block, aTNA, are very stable, do not degrade in the blood, have been shown to be non-toxic to cells, and do not elicit a nonspecific immune response.
When the researchers coupled a particular type of biomolecule, which is known to bind to a biomarker in high-specificity breast cancer cells, to the new 4WJ structure, it turned out that the 4WJ structure may prove effective in directing cancer drugs to the desired cells.
In addition, by making further modifications to the new 4WJ structure, they could extend its lifetime in the bloodstream and thus also the effect of the drug that may be coupled to the DNA nanostructure.
The researchers imagine that their 4WJ structure built with artificial building blocks can both be used as a tool to transport drugs to the right position in a patient's body. In addition, they see that it can serve as a valuable tool in research. For example, researchers imagine that the effects of different combinations of cancer-degrading biomolecules can be screened faster and more efficiently, so that the most effective cancer treatment can be found more quickly.
Center for Multifunctional Biomolecular Drug Design (CEMBID)
The Center for Multifunctional Biomolecular Drug Design (CEMBID) was funded by the Novo Nordisk Foundation with DKK 60 million in 2018 and runs until 2024.
Professor Kurt V. Gothelf heads the center with a background in organic chemistry, bioconjugation and DNA nanotechnology, but the interdisciplinary team of researchers at CEMBID works in the fields of chemistry, molecular biology, pharmacology and medicine.
The colleagues from iNANO: Professor Jørgen Kjems and Associate Professor Ken Howard help lead the center, where Jørgen Kjems has great expertise in nucleic acid nanotechnology and drug delivery, while Associate Professor Ken Howard is an expert in drug delivery and pharmacokinetics. Also, from Vrije Universiteit in Brussels is Professor Tony LaHoutte, who through his extensive experience with clinical trials of bioconjugates contributes in connection with testing of the new coupled drugs.
CEMBID’s website: www.cembid.au.dk
SUPPLEMENTARY INFORMATION, INCLUDING CONTACT INFORMATION
We strive to ensure that all our articles live up to the Danish universities' principles for good research communication. Against this background, the article is supplemented with the following information:
ITEMS | CONTENT AND PURPOSE |
External funding | Novo Nordisk Foundation (CEMBID, NNF17OC0028070) |
Conflicts of interest | The researchers declare that there are no conflicts of interest. |
Link to scientific paper | Functionalized Acyclic (L)-Threoninol Nucleic Acid Four Way Junction with High Stability in Vitro and in Vivo. |
Contact information | Professor Kurt Gothelf |
