New high-throughput screening study may pave the way for future Parkinson’s disease therapy
Parkinson's disease is the most common neurodegenerative disease; currently there is no cure. Aggregation of the protein α-synuclein plays a key role in this disease. Together with a US drug company, AU researchers have now carried out a new screening strategy which has identified novel and structurally diverse aggregation inhibitors.

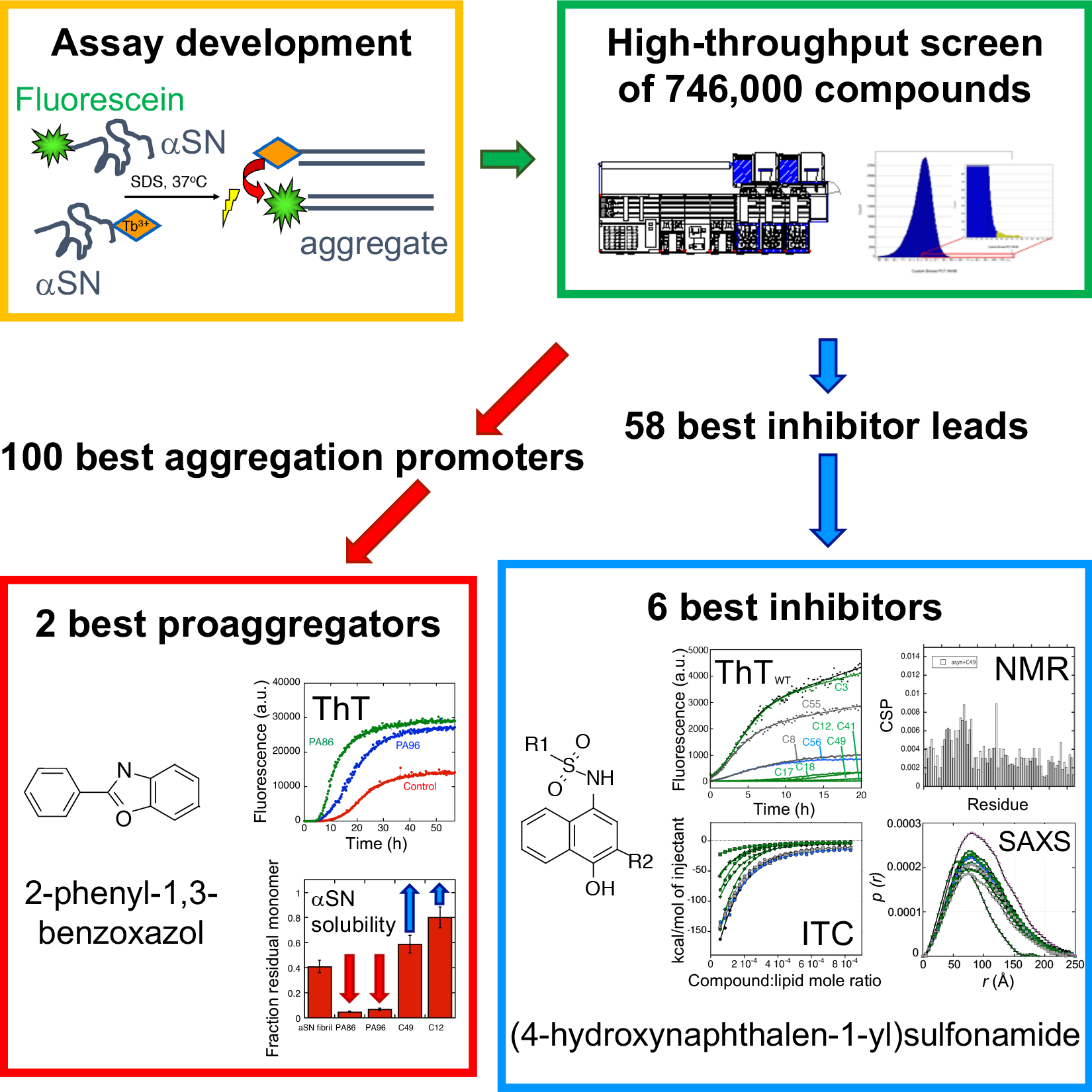
Parkinson's disease (PD) is the most common movement disorder in the world. PD patients suffer from shaking, rigidity, slowness of movement and have difficulties walking. It is a neurodegenerative disease and is caused by the loss of dopaminergic neurons in the brain. Currently PD cannot be cured or even halted in its development, but symptoms may be treated to some degree. Probably the single most important cause of PD is the aggregation of the natively unfolded protein a-synuclein (αSN). αSN can form both small oligomeric complexes (αSOs) as well as large fibrillary deposits; the αSOs are thought to be the most toxic species. If we can prevent or reduce αSN aggregation we have a good way to halt PD development. So far it has been difficult screen large numbers of compounds to identify potential aggregation inhibitors, since αSN aggregates in a rather irregular and variable fashion; it is also difficult to detect early-stage αSOs.
However, in the new screening strategy, the researchers first developed a smart trick to make αSN aggregate in a more predictable way using the “soap” molecule sodium dodecyl sulfate. To detect the aggregates, they then used Förster Resonance Energy Transfer (FRET), a widely used technique for measuring distances within and between molecules. In this way they were able to screen 746,000 compounds for their ability to inhibit αSN aggregation. By sifting through the results, they came up with a collection of structurally diverse, novel small compounds that either prevent (inhibitor) or accelerate (proaggregator) αSN aggregation. The six best inhibitors share a common core structure and these compounds all interact with the first part of αSN, called the N-terminal region.
The results are exciting in two ways. Firstly, the identified inhibitory molecules could be useful starting points to develop therapy against PD. Secondly, the compounds can also be used to find out more about how αSN aggregation in the cell affects PD development and thus understand more about the molecular basis for PD.
This work was financially supported via the innovation consortium CureND by Danish Ministry of Science, Technology, and Innovation, the councils FNU, FTP and FSS from Independent Research Fund Denmark, the Danish National Research Foundation (Center of Excellence inSPIN) as well as Aarhus University Research Foundation.
The research has been carried out by scientists from Interdisciplinary Nanoscience Centre (iNANO) and Department of Molecular Biology and Genetics at Aarhus University (AU) in collaboration with Wyeth Research (now part of Pfizer). Professor Daniel Otzen has been in charge of the research team behind the study.
For further information, please contact
Professor Daniel Otzen
Interdisciplinary Nanoscience Center and Department of Molecular Biology and Genetics
dao@inano.au.dk – +45 20725238
