Energy storage: Hydrogen can be packed extremely tightly in a nanoporous material
Researchers at Aarhus University have discovered a porous material in which hydrogen can be packed twice as densely as in pure liquid hydrogen. This is because hydrogen forms penta-molecular units that have not previously been observed. Hydrogen also forms a new type of bond with the lattice structure, called a hexahydrogen bond. This discovery opens new perspectives for efficient and safe energy storage.
In a recent scientific paper published in Nature Chemistry, an international research team led by Aarhus University, Université Catholique de Louvain, Belgium, and the Max Planck Institute, Stuttgart, presents a significant development in energy storage. Using a nanoporous material based on magnesium borohydride, γ-Mg(BH4)2, the researchers have achieved a denser packing of hydrogen molecules than previously seen. This may inspire new ways to store and transport hydrogen, a key component in the transition to a 'greener' energy system.
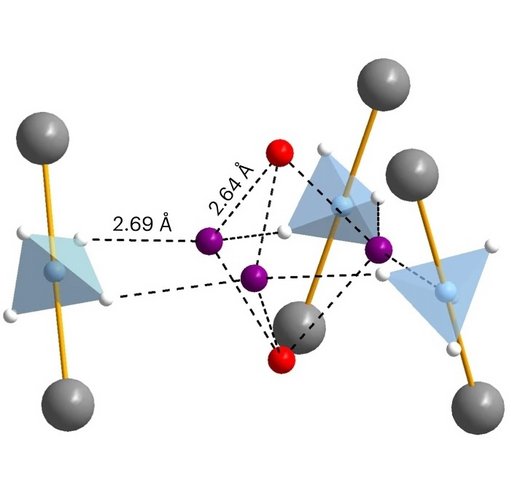
Using advanced neutron scattering experiments, it has been possible to clarify the exact atomic structure and see exactly how the hydrogen molecules are organised in the nanoporous material. It is very surprising that hydrogen molecules are adsorbed in different positions than nitrogen and that there is room for 3.5 times more hydrogen than nitrogen. This is because the hydrogen molecules can form penta-dihydrogen clusters that have not been observed before.
In penta-dihydrogen clusters, the hydrogen molecules bind in two different ways. At the position that fills first, they bind with weak non-directional van der Waals interactions that allow the molecules to rotate freely around their centre of mass.
As more hydrogen is adsorbed, the next position is filled, where hydrogen binds to the nanoporous material. The research team has discovered that this is a new type of directional electrostatic bonding. The hydrogen molecules at this position cannot rotate freely, but are fixed by four other hydrogen atoms in the lattice structure. Macroscopic volumetric measurement of gas adsorption has confirmed that there are two different hydrogen positions in the solid and that one position is filled before the next. The nanoporous material can absorb an extreme amount of hydrogen corresponding to the composition, γ-Mg(BH4)2×2.33H2. This means that hydrogen is twice as densely packed in the porous material as in pure liquid hydrogen.
Hydrogen has been the focus of renewable energy storage for decades because it has by far the highest energy content by weight. On the other hand, it has a low volumetric energy content. Therefore, there has previously been a lot of focus on adsorbing hydrogen in nanoporous materials. However, it has not previously been possible to get as much hydrogen into porous materials as in this case. Nor has it been possible to determine exactly how the adsorbed molecules are organised and bound to the porous material to be packed so extremely tightly.
These new discoveries provide a fundamental scientific understanding of how hydrogen can be bound in porous matter and provide inspiration for the development of new materials for hydrogen storage in a solid.
About the research
Study type:
Experimental chemistry
External funding:
H.O. is grateful for partial funding by the National Research Foundation of Korea (NRF) (grant 2022R1A2C3005978). Partial funding by the German Research Foundation (DFG) within the priority programme SSP 1362 is gratefully acknowledged. We thank the F.R.S.-FNRS (Belgium), the Fonds Spéciaux de Recherche (UCL), Fédération Wallonie-Bruxelles (ARC 18/23-093 MicroBat) and General Motors Global R&D for financial support. We acknowledge the support of NIST, the US Department of Commerce and Helmholtz-Zentrum Berlin in providing the neutron research facilities used in this work. Neutron beam time at Helmholtz-Zentrum Berlin was awarded via funding proposal CHE-01-3161. The work was supported by The Danish Research Council for Nature and Universe (Danscatt) and by The Independent Research Fund Denmark for Technology and Production (9041-00226B). We are grateful to the Carlsberg Foundation and China Scholarship Council for the fellowship of X. Li (CSC201706370133). The neutron scattering experiment was performed at ORNL’s Spallation Neutron Source, supported by the Scientific User Facilities Division, Office of Basic Energy Sciences, US Department of Energy, under contract no. DE-AC0500OR22725 with UT Battelle. R.B.-X. gratefully acknowledges research support from the Hydrogen Materials—Advanced Research Consortium (HyMARC), established as part of the Energy Materials Network under the US Department of Energy, Office of Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cell Technologies Office, under contract no. DE-AC05-00OR22725. S.P.-P. acknowledges a Marie Curie fellowship (H2020-MSCA-IF-2020-101020330). Computational time at the MARENOSTRUM supercomputer was provided by the Barcelona Supercomputing Centre through a grant from Red Española de Supercomputación (QHS-2022-3-0003).
Conflicts of interest:
The researchers declare that there are no conflicts of interest in connection with this research.
Link to the scientific article:
Small-pore hydridic frameworks store densely packed hydrogen
Hyunchul Oh, Nikolay Tumanov, Voraksmy Ban, Xiao Li, Bo Richter, Matthew R. Hudson, Craig M. Brown, Gail N. Iles, Dirk Wallacher, Scott W. Jorgensen, Luke Daemen, Rafael Balderas-Xicohténcatl, Yongqiang Cheng, Anibal J. Ramirez-Cuesta, Michael Heere, Sergio Posada-Pérez, Geoffroy Hautier, Michael Hirscher, Torben R. Jensen & Yaroslav Filinchuk
Nature Chemistry, 2024, 1-16 (6 February 2024) https://www.nature.com/articles/s41557-024-01443-x
Contact information:
Professor Torben René Jensen
Aarhus University
Institut for Kemi og Interdisciplinært Nanoscience Center (iNANO)
Email: trj@chem.au.dk