New drug design may be a step towards developing long-awaited immunotherapy against prostate cancer
Despite numerous trials in the past decade prostate tumors have so far resisted treatment with immunotherapy. Now iNANO and Aarhus University Hospital researchers join forces to move prostate cancer immunotherapies closer to the clinic by using Albumin-fused T-cell engagers as a potential immunotherapy tailored to selected prostate cancer patients.
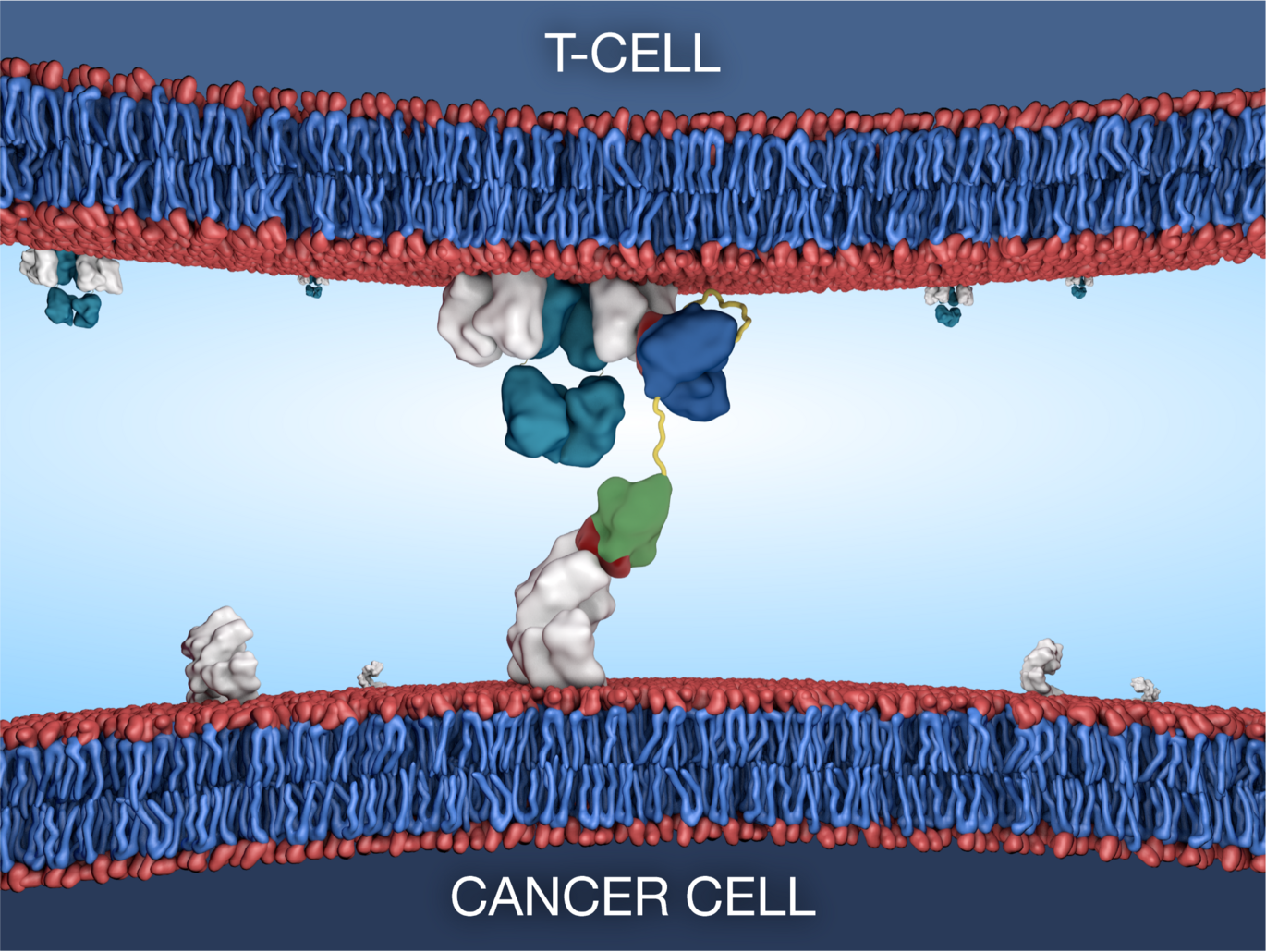


Multiple cancers have seen long-term death rate declines, particularly with the advent of exciting new immunotherapeutic drug types. Prostate cancer, however, remains the second leading cause of cancer death in men in Western countries with immunotherapy yet to be successfully adopted with multiple clinical trials failing to impact clinical practice.
Bispecific T-cell engaging antibodies offer a potential solution by utilising the patients’ own immune response by linking T-cells to cancer cells with concurrent killing of the cancer cell. However, these drug types investigated for use in prostate cancer are plagued by drug design flaws and varied response rates between patients.
Identification of subsets of patients who will most likely benefit from treatment and improving the drug format has the potential to pave the way for increased drug response and higher clinical success rates.
Joining forces with clinical scientists
The Bioengineered Drug Design Laboratory at iNANO led by Associate Professor Ken Howard, have recently applied their albumin-fusion platform to develop a new bispecific antibody that targets the prostate specific membrane antigen (PSMA) commonly overexpressed in malignant prostate tissue. The design exists in different formats allowing for tunable effects through programmable interaction with the neonatal Fc receptor.
Through collaboration with The Prostate Cancer Research Group at Department of Molecular Medicine, Aarhus University Hospital, led by Professor Karina Dalsgaard Sørensen, an aggressive prostate cancer patient subset was identified with high T-cell infiltration and high expression of PSMA, both characteristics that make them ideal candidates for treatment with the novel bispecific T-cell engager.
Novel immunotherapy strategies may save lives
The translational findings have been published in British Journal of Cancer (cf. link below) first-authored by Eske N. Glud from the research group of Ken Howard. In collaboration with Karina Dalsgaard Sørensen they unite fundamental and clinical science towards development and implementation of more effective treatment for prostate cancer.
Ken Howard “We hope that this work will inspire Pharma to adopt and develop these new types of drug designs for more potent and tailored immunotherapy”
Karina Dalsgaard Sørensen “Development of novel immunotherapy strategies tailored to patients with aggressive prostate cancer subtype could save many lives in the future. Our results are very promising, but we are also aware that the road to clinical implementation of new drugs is long but this work is a step in the right direction.”
SUPPLEMENTARY INFORMATION
We strive to ensure that all our articles live up to the Danish universities' principles for good research communication. Against this background, the article is supplemented with the following information:
Study type:
- Experiment
External funding:
- Novo Nordisk Foundation (CEMBID, NNF17OC0028070)
- Independent Research Fund Denmark (6110-00450B and 9039-00084B)
Conflicts of interest
- The researchers declare that there are no conflicts of interest.
Link to scientific papers
Contact information
Associate Professor Ken Howard
Interdisciplinary Nanoscience Center (iNANO) & Department of Molecular Biology & Genetics
Aarhus University
Email: kenh@inano.au.dk
Professor Karina Dalsgaard Sørensen
Department of Clinical Medicine
Aarhus University Hospital
Email: kdso@clin.au.dk
