ELECTROCHEMICAL ANALYSIS OF THE FIBRILLATION OF PARKINSON'S DISEASE ?-SYNUCLEIN
Amyloid formation of proteins and peptides play a crucial role in many diseases such as Alzheimer’s, Diabetes and Parkinson’s disease. This makes amyloid formation a research field of high importance although it is not yet fully understood. Hence the development of fast and reliable characterization methods for real-time monitoring of protein misfolding is crucial if detection or treatment is to be carried out successfully.
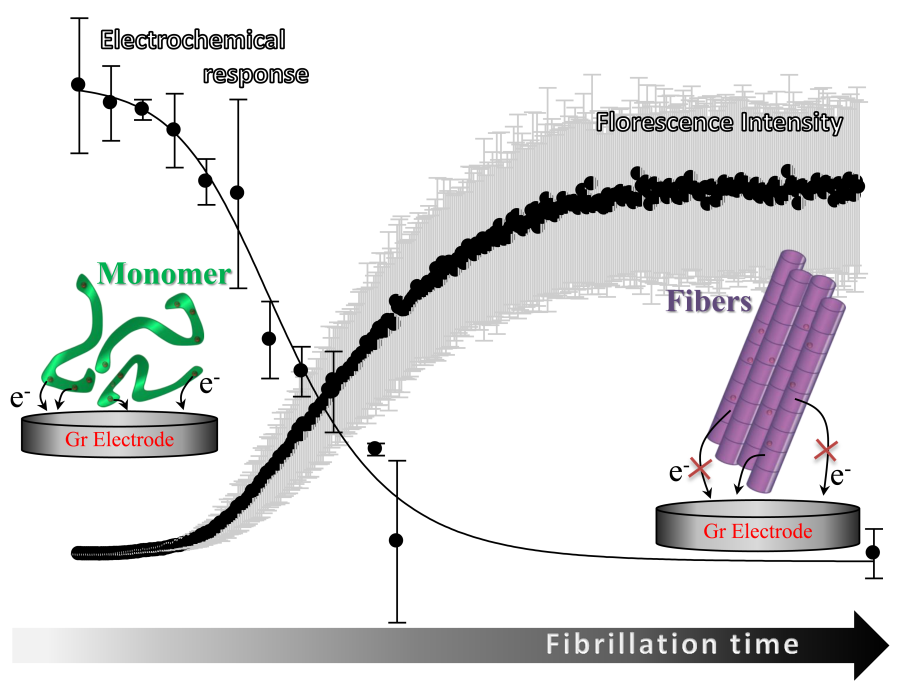
Now a group of iNANO scientists (from the groups of Elena Ferapontova and Daniel Otzen) have developed a new way of detecting the extent of aggregation of ?-synuclein (?SN), involved in Parkinson's disease and other neurodegenerative disorders by monitoring the electrochemical oxidation of tyrosine (Tyr) residues ?SN.
This allows for an easy way of detection and monitoring small monomeric species of ?SN which are believed to be the toxic species in the overall fibrillation pathway. This new technique will serve as a great supplement to fluorescence which is best used for monitoring fibers and larger molecules. Now researchers can easily follow the whole process in vivo from monomer to fibril.
Their research article entitled “Electrochemical analysis of the fibrillation of Parkinson’s disease ?-synuclein” is recently published in the Analyst
