Ground breaking study on membrane protein folding
Most detailed description of how membrane protein folds
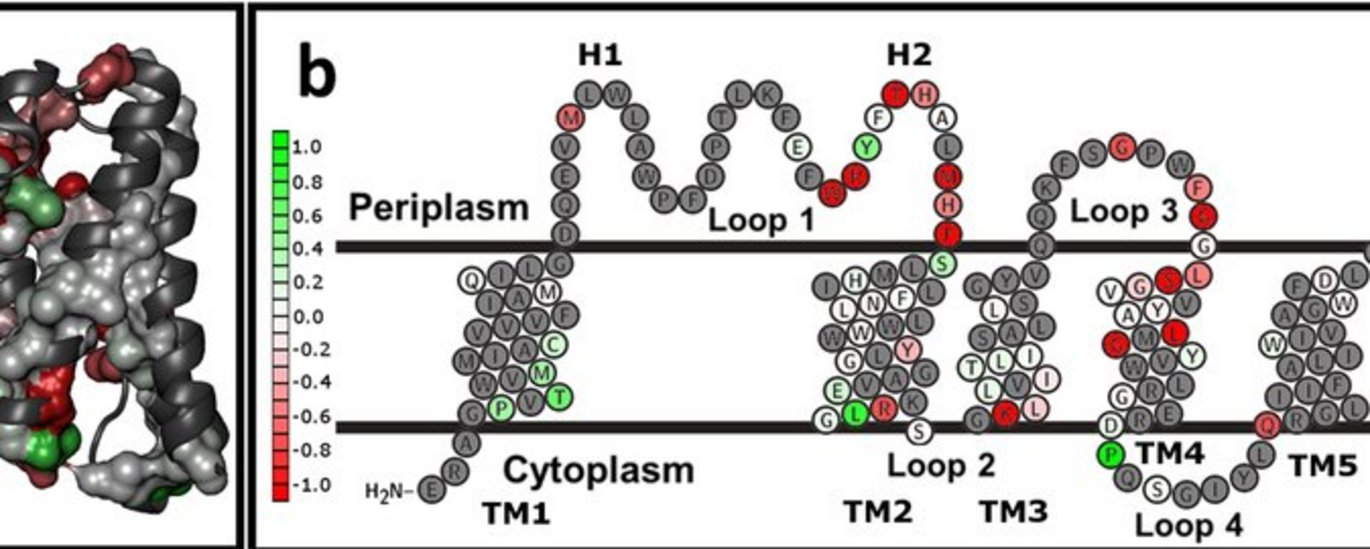
For the last three decades, intense work has been carried out to understand how proteins fold, i.e. how they achieve their fine three-dimensional structure – which is also the basis for their biological function, regardless of whether it concerns metabolism, signal transfer or structure. The greatest progress by far has been made in water-soluble proteins, which are generally quite straightforward to work with.
Read more (in Danish only).
Authors:
Paslawski, W., Kristensen, J. V., Lillelund, O., Schafer, N., Baker, R., Urban, S., and Otzen, D. E. (2015) Cooperative folding of a polytopic α-helical membrane protein involves a compact N-terminal nucleus and non-native loops, Proc. Natl. Acad. Sci. USA8. June 2015.
For more information, please contact
Professor Daniel Otzen
iNANO
dao@inano.au.dk
+45 2072 5238
