Controlling the conversion of CO2 with new catalysts
Converting CO2 into more useful products offers an interesting strategy to mitigate climate issues while producing value added chemicals. Researchers from the Carbon Dioxide Activation Center (CADIAC) have discovered how the ligand scaffold around a manganese catalyst can be modified to control the selectivity of the products generated via conversion of CO2.
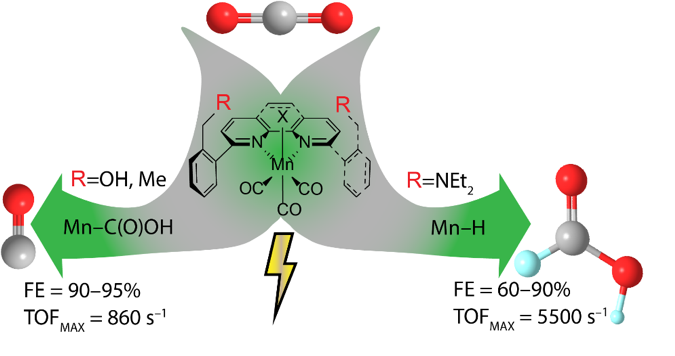

Global warming caused by anthropogenic CO2 emissions is one of the most urgent challenges, and the need for developing strategies for green production of fuels and chemicals is great. Transforming CO2 to useful chemicals represents a promising solution and for this selective electrochemical reduction is an attractive method as it could be driven by solar, wind, or hydropower.
In a recent article from Carbon Dioxide Activation Center (CADIAC), the research groups of Kim Daasbjerg, Troels Skrydstrup, and Mu-Hyun Baik (Korean Advanced Institute of Science and Technology, KAIST) collaborated to design a range of engineered catalysts for the reduction of CO2. The reduction products can be tuned to either produce preferentially carbon monoxide or formic acid depending on small structural changes to the ligand surrounding the catalysts. Interestingly, the designed catalysts are also among the most active molecular catalysts for the conversion of CO2 to formic acid.
This study highlights the importance of an interdisciplinary collaboration combining chemical synthesis, electrochemical techniques and calculations to design and understand the catalyst activity.
More Information:
The research was carried out by scientists from the Interdisciplinary Nanoscience Centre (iNANO) and the Department of Chemistry, Aarhus University (AU) in collaboration with Professor Mu-Hyun Baik from the Korea Advanced Institute of Science and Technology (KAIST).
The work was generously supported by the Danish National Research Foundation (DNRF118), NordForsk (grant no: 85378), and the Institute for Basic Science in Korea (IBS-R010-A1)
Read more about the results in Journal of the American Chemical Society:
For further information, contact:
Professor Kim Daasbjerg, email: kdaa@chem.au.dk
Professor Troels Skrydstrup, email: ts@chem.au.dk
Interdisciplinary Nanoscience Center
Department of Chemistry
Aarhus University
Denmark
