Publication in Eur. J. Org. Chem!
Check out our latest publication by PhD student Julia on trifluoromethylthiolations!
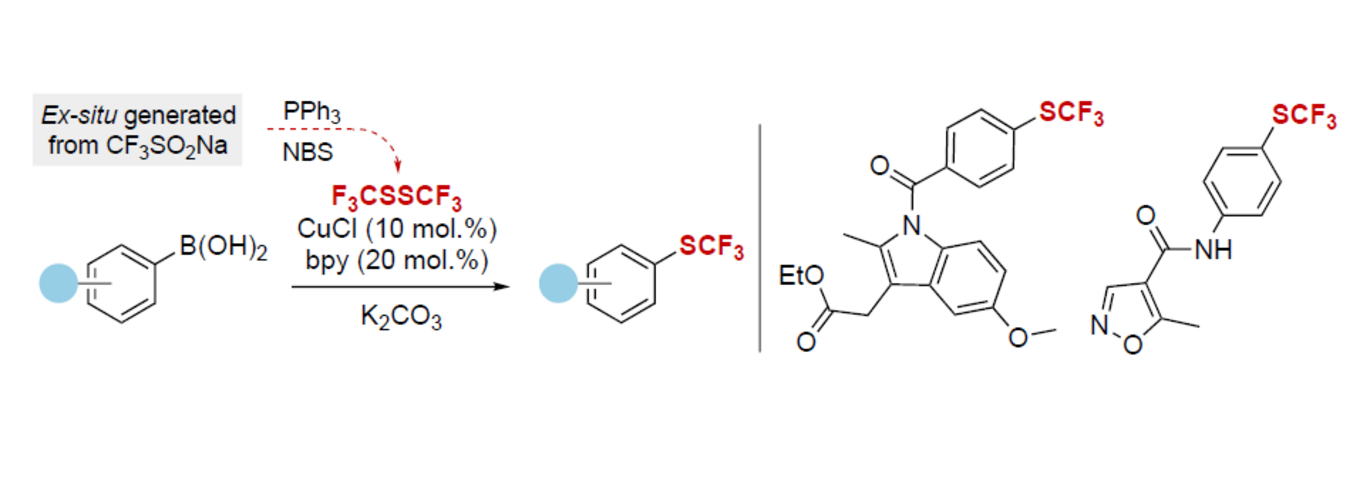
Abstract: Herein, a convenient and operationally simple protocol for the ex-situ generation of bis(trifluoromethyl)disulfide from the readily available and commercial Langlois reagent is reported. The one-step synthesis of the toxic and volatile CF3SSCF3 is performed in a two-chamber reactor with simple PPh3 and N-bromosuccinimide as the activator, allowing for the safe handling and tandem utilization in direct trifluoromethylthiolation reactions. The versatility of the ex-situ generated CF3SSCF3 is demonstrated in known electrophilic, nucleophilic, and a radical trifluoromethylthiolation reactions. Furthermore, the application of the CF3SSCF3 in a copper-catalyzed cross-coupling with boronic acids is disclosed, showing good to excellent yields of trifluoromethyl-substituted aryl products, including pharmaceutically relevant molecules.
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/ejoc.202300843