Green methanol production: Visualizing methanol synthesis catalyst surface dynamics
A research team from iNANO, led by Prof. Jeppe V. Lauritsen, has made discoveries about the industrial catalyst used to convert captured CO₂ and H₂ into green methanol—an essential component in the transition to sustainable energy. Their findings, published in Nature Communications, provide unprecedented insights into the surface dynamics of this catalyst under realistic operating conditions.
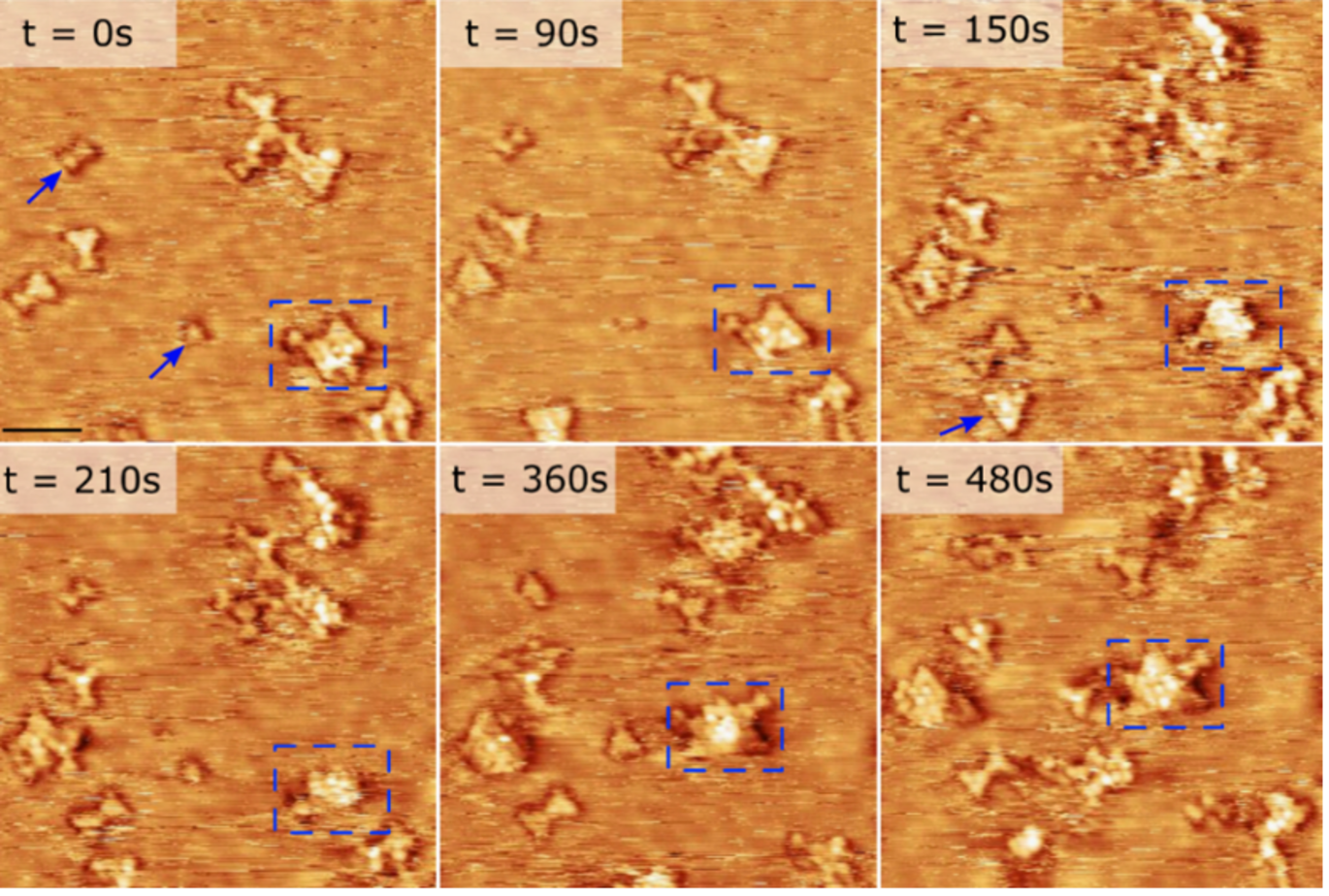
Unveiling catalyst surface reorganization
Using state-of-the-art high-resolution scanning tunneling microscopy, the team captured detailed images of the catalyst surface under reactive conditions. This advanced imaging technique allowed the researchers to observe how the presence of carbon monoxide (CO) in the reaction gas induces significant reorganization of the active surface. Specifically, they discovered that Zn atoms, which are initially located within the surface of a Cu alloy, migrate to the surface when CO is introduced alongside CO₂ and H₂. This surface restructuring is crucial because it directly influences the catalyst's efficiency and stability.
Implications for industrial methanol production
The observations made by the iNANO team have significant implications for the industrial production of methanol. By understanding the exact behavior of the catalyst at the atomic level, scientists and engineers can develop more accurate models of the catalyst's functionality. This knowledge is essential for optimizing the operational conditions of methanol synthesis, potentially leading to more efficient and sustainable production processes.
The reorganization of Zn atoms suggests that the presence of CO not only affects the catalyst's surface structure but also its catalytic properties. These findings could lead to the development of new strategies to enhance catalyst performance, such as fine-tuning the composition of reaction gases or modifying the catalyst's surface structure to improve its reactivity and longevity.
Future directions and potential applications
The study opens up new avenues for further research into catalyst behavior under real-world conditions. Future studies could explore how other components in the reaction mixture affect the catalyst's surface dynamics or investigate the long-term stability of the restructured catalyst. Additionally, the insights gained from this research could be applied to other catalytic processes beyond methanol synthesis, potentially benefiting a wide range of industrial applications.
Prof. Lauritsen and his team's work exemplifies the cutting-edge research being conducted at iNANO. It highlights the center's commitment to addressing global energy challenges through innovative nanoscience solutions.
About the research
Study type:
Experimental physics
External funding:
Grants from the Independent Research Fund Denmark (Grant no. 9041-00070B) and Villumfonden (Grant no. 13264) are acknowledged (J.V.L.).
Conflicts of interest:
The authors declare no competing interests.
Link to the scientific article:
Visualizing the gas-sensitive structure of the CuZn surface in methanol synthesis catalysis
Sigmund Jensen, Mathias H. R. Mammen, Martin Hedevang, Zheshen Li, Lutz Lammich & Jeppe V. Lauritsen
Nature Communications, 2024, 15, 3865, https://www.nature.com/articles/s41467-024-48168-6
Contact information:
Professor Jeppe Vang Lauritsen
Aarhus University
Interdisciplinary Nanoscience Centre (iNANO) and Department of Physics & Astronomy
Email: jvang@inano.au.dk
