Albumin solution to an unmet need in cancer immunotherapy
AU researchers join forces with world leaders to develop a new albumin-based platform for long-acting, efficient, and safer immunotherapy. Assoc. Prof. Ken Howard and his research team have published their findings in a new Nature family journal.

Cancer immunotherapy aims to simulate the patient’s own immune defence to attack cancer cells.
Bispecific antibodies are a drug attractive comprising of a single molecule fusion of an antibody that binds the patient T-cells and the other the cancer cell. Bringing the T-cell and cancer cell in close proximity results in T-cell destruction of the cancer.
Current T-cell engaging bispecific antibodies have a short blood circulatory half-life, meaning they are quickly removed from the body. The patient, therefore, requires high and frequent dosing of the immunotherapy, which can lead to tissue damage and infusion reactions.
Consequently, there is a need for safe strategies to control the circulation in the bloodstream of the immunotherapy.
Joining forces to engineer immunotherapies with a programmable half-life
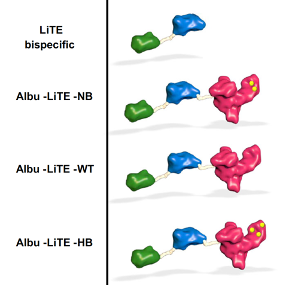
Associate Professor Ken Howard and his research team in the Bioengineered Drug Designs Lab have worked together with global leaders, Luis Alvarez-Vallina from the University Hospital in Madrid and Jan Terje Andersen at the University of Oslo to develop T-cell engaging bispecific antibodies with tunable half-life. The collaboration has resulted in a paper first-authored by Ole Mandrup in a Nature family journal.
Howard’s research focuses on albumin, the most abundant protein in human blood with a long circulatory half-life due to engagement with the neonatal Fc receptor (FcRn) and the use of albumins engineered with different affinity for FcRn as a method to control the movement of albumin drugs in the body. What the researchers did was to engineer constructs of T-cell engaging bispecific antibodies created by Luis Alvarez-Vallina (LiTE) fused to. The hypothesis was that the albumins would extend the half-life of the overall construct (Albu-LiTE).
In fact, the circulation time of the Albu-LiTE constructs was shown to be programmable by the albumin type and its FcRn affinity, which allows fine-tuning of the circulatory half-life. A proof-of-concept was provided in a humanized albumin/FcRn AlbuMus mouse.
Killing cancer cells in new AlbuMus mouse strain
Therapeutic evaluation was performed in a world’s first immunocompromised version of the AlbuMus developed by the Howard lab for testing albumin-based anti-cancer drugs.
Human cancer cells were injected into the mice and the lack of an immune system ensured that the cells were not rejected by the mouse’s body. The Albu-LiTE construct was injected into the mice along with immune cells isolated from a human volunteer. The researchers observed that only one dose of the AlbuLiTE therapy was required to inhibit the growth of the tumors in contrast to conventional therapies.
The potential benefit of the extended half-life is long-acting effects as well as lower and less frequent doses given to the patient.
“Albumin offers a safe alternative to present half-life extension technologies that can be engineered into the design of bispecific antibodies to meet an unmet challenge in the field. We have applied our albumin expertise in the establishment of an immunotherapy platform at iNANO and within CEMBID that allows programmable half-life to tailor pharmacokinetics to maximise therapeutic efficacy and safety,” Says Ken Howard.
Read more about the results in Nature Communications Biology:
The research was carried out by researchers from Interdisciplinary Nanoscience Center (iNANO), the Department of Molecular Biology and Genetics, and Department of Biomedicine (Aarhus University), University of Oslo, and University Hospital of Madrid. The work was financially supported by the Novo Nordisk Foundation (Center for Multifunctional Biomolecular Drug Design, CEMBID) and the Spanish Ministry of Economy and Competitiveness.
For further information, please contact:
Associate Professor Ken Howard
Interdisciplinary Nanoscience Center (iNANO)
Aarhus University
Email: kenh@inano.au.dk
