Efficient and mild synthesis of pharmaceutically relevant chemical motif
Danish-Belgian collaboration headed by Aarhus University professor, Troels Skrydstrup, is the source of a an improved way to create certain flourine-containing substructures, which have shown several beneficial medical properties.

When introducing a fluorine atom into bioactive molecules it its possible to specifically change their chemical and biological properties. The number of fluorine-containing drugs being launched is increasing, which makes it relevant striving to develop new synthetic methodologies for incorporation of fluorine-containing motifs. a,a-bis(trifluoromethyl)carbinol group is a motif in this category and compounds containing this substructure have shown biological activity against cancer, diabetes, hepatitis C, dyslipidemia and inflammation. The large number of fluorine atoms in the motif also makes promising contrast agents for functional magnetic resonance imaging (fMRI), which can be used for measuring brain activity.
Even though the impact of this substructure is multidisciplinary, there are only few reports demonstrating the installation of the motif and common characteristics of these methods are that they are inefficient and include toxic reagents. Consequently, there is an incentive to seek a general and mild protocol for the direct conversion of aryl bromides or fluorosulfates into (hetero)-aryl a,a-bis(trifluoromethyl)carbinols.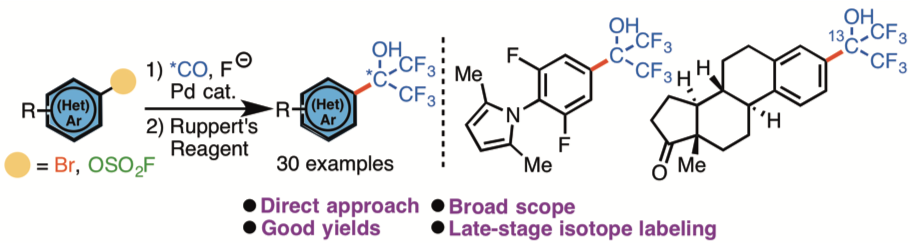
Figure: The direct transformation of aryl bromides and fluorosulfates to their corresponding aryl Bis(trifluoromethyl)carbinols. (by Troels Skrydstrup)
The research team has applied transition metal catalysis to introduce an important fluorine-containing motif to an aromatic core starting from an aryl halide. The so-called bis(trifluoromethyl)carbinol group represents an important bioisostere of a carboxylic acid and there is a general interest from the pharmaceutical industry for the installation of this functional group in bioactive molecules, as its acidity is relatively low and the group displays a great lipophilicity than carboxylic acids.
What is particular novel in the study is that the transformation was achieved through the use of the C-1 building block, carbon monoxide, to generate an intermediate acid fluoride followed by double addition of Ruppert’s reagent.
The results are exciting both from an experimental and therapeutic point-of-view as this chemistry will undoubtedly allow for the rapid introduction of the bis(trifluoromethyl)carbinol unit into a wide variety of pharmaceutically relevant molecules.
This work was generously supported by the Danish National Research Foundation (grant no. DNRF118).
The research has been carried out by researchers from Aarhus University (Department of Chemistry and Interdisciplinary Nanoscience Centre) and Molecular Design and Synthesis, Department of Chemistry at Katholieke Universiteit Leuven. Troels Skrydstup, Professor at Aarhus University, has been in charge of the research team behind the study just published in the prestigious journal Angewandte Chemie International Edition.
Direct Access to Aryl Bis(trifluoromethyl)carbinols from Aryl Bromides or Fluorosulfates via a Pd-Catalyzed. Carbonylation. Katrine Domino, Cedrick Veryser, Benjamin A. Wahlqvist, Cecilie Gaardbo, Karoline T. Neumann, Kim Daasbjerg, Wim M. De Borggraeve and Troels Skrydstrup*. doi: 10.1002/anie.201802647.
For further information, please contact
Professor Dr. Troels Skrydstrup
Interdisciplinary Nanoscience Center
Department of Chemistry
Aarhus University
Denmark
Tel: +45 28992132
Email: ts@chem.au.dk , ts@inano.au.dk
