| | Ultra Efficient and Selective CO2-to-CO Conversion.
Electrochemical and Photochemical Molecular Catalysis of the Reduction of CO2 in Aprotic Solvent and Pure Water Using Fe(0) Porphyrin Catalysts. Derivatives of iron tetraphenylporphyrin bearing prepositioned phenolic functionalities on two of the opposed phenyl groups prove to be remarkable catalysts of the reduction of CO2 to CO when generated electrochemical at the Fe(0) oxidation state, both in terms of selectivity (the CO faradaic yield is nearly quantitative), durability, overpotential and turnover frequency in DMF-water mixtures.(1-5) Based on cyclic voltammetry and quantum calculations, mechanisms for reduction were fully deciphered. (3-4) Benchmarking with other catalysts, through catalytic Tafel plots, shows that they are the most efficient homogeneous molecular catalysts of the CO2-to-CO conversion at present. (5) Extending these studies, we have been able to catalyze CO2 reduction with visible light as a source for energy, (6) a remarkable output for a system that combines an abundant metal (iron) based catalyst, a cheap organic sensitizer and visible photons. 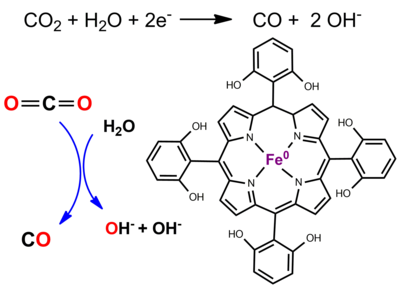
Recently, we further discovered that it was possible, with a water-soluble Fe porphyrin, to catalyze the electrochemical conversion of carbon dioxide into carbon monoxide in aqueous solution. The reaction, performed in pH-neutral, unbuffered water, forms quasi-exclusively carbon monoxide with very little production of hydrogen, thus paving the way to a complete CO2-to-CO conversion cell by immobilizing the catalyst onto the electrode surface by means of a negatively charged polymer and association with a water-oxidation anode through a proton-exchange membrane. (7) References
(1) S. Drouet, C. Costentin, M. Robert, J-M. Savéant, Science, 2012, 338, 90-94.
(2) C. Costentin, M. Robert, J-M. Savéant, Chem. Soc. Rev., 2013, 42, 2423-2436.
(3) C. Costentin, G. Passard, M. Robert, J-M. Savéant, J. Am. Chem. Soc., 2013, 135, 9023-9031.
(4) C. Costentin, G. Passard, M. Robert, J-M. Savéant, J. Am. Chem. Soc., 2014, 136, 11821-11829.
(5) C. Costentin, G. Passard, M. Robert, J-M. Savéant, PNAS, 2014, 111, 14990-14994.
(6) J. Bonin, M. Robert, M. Routier, J. Am. Chem. Soc., 2014, 136, 16768-16771.
(7) C. Costentin, M. Robert, J-M. Savéant, A. Tatin, PNAS, 2015, in press.
|


