New chemical methods by the Gothelf Lab with potential for drug delivery
Traditional medicines typically work by targeting the body in a non-specific way, which can lead to unnecessary side effects and the need for taking more medicine than necessary. Researchers from iNANO and Department of Chemistry have developed new chemical methods, which may prove useful in targeted drug delivery.
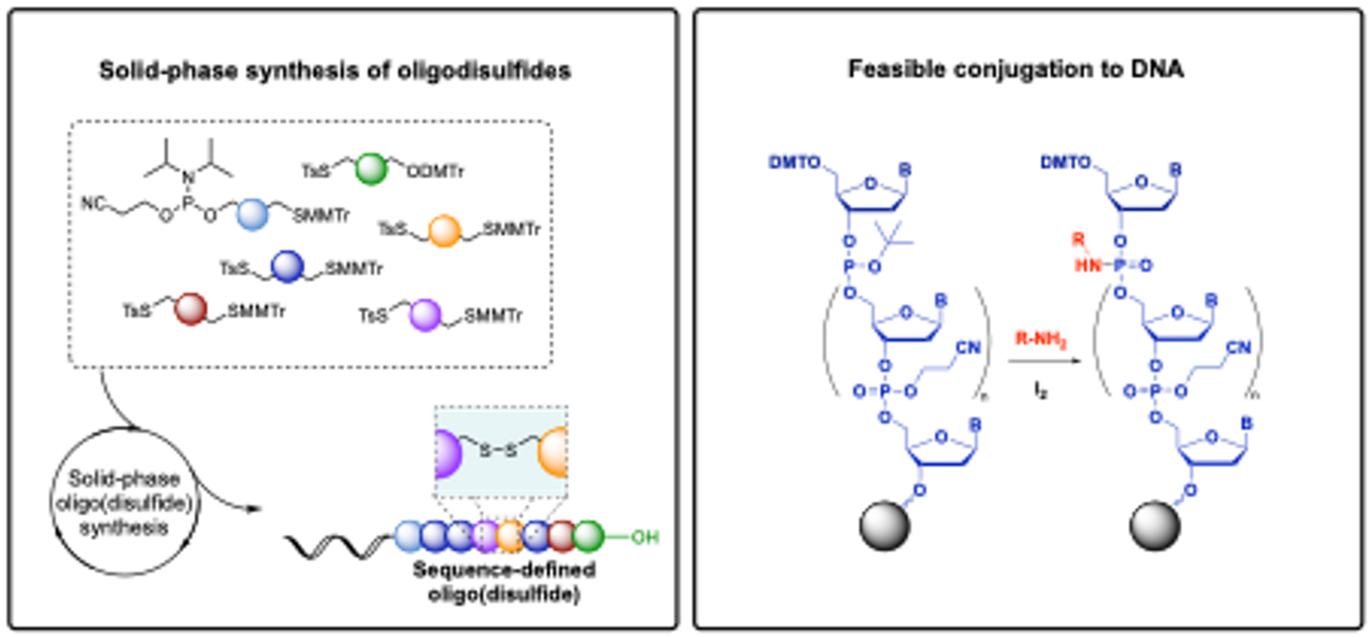
By using targeted drug delivery, the aim is to potentiate the therapeutic effect of medicinal drugs by delivering pharmaceuticals in specific organs, tissues, or cells. Recently, two papers from the Gothelf Lab have been published in the scientific journal Angewandte Chemie, which display the development of new chemical methods with potential use for drug delivery.
More specifically, the papers detail the development of new methods in automated solid-phase synthesis of sequence-defined oligomers.
In the first paper, Martin Frandsen, Alexander Sandahl, and their colleagues describe a novel method for synthesizing sequence-specific disulfide oligomers. This class of oligomers has major potential for drug delivery and for modifying oligonucleotides with cleavable targeting, delivery, and therapeutic disulfide oligomers.
In the second paper, Rikke Hansen and her team have developed a feasible and inexpensive method to functionalize oligonucleotides with chemical handles during the solid-phase synthesis of oligonucleotides.
This process can be achieved using only an amine and iodine, and has the potential for conjugation to drugs, dyes, and biomolecules. Additionally, the phosphoramidate linkage is acid-sensitive and can be cleaved at lower pH levels. The cleavage reaction was successfully demonstrated for a DNA-antibody conjugate, among other compounds. This approach holds promise for drug delivery by cleavage of DNA conjugates in the acidic lysosomes following cellular uptake.
ITEMS | CONTENT AND PURPOSE |
External funding | Novo Nordisk Foundation (CEMBID, NNF17OC0028070) |
Conflicts of interest | The researchers declare that there are no conflicts of interest. |
Link to scientific papers | Automated Solid-Phase Oligo(disulfide) Synthesis Insertion of Chemical Handles into the Backbone of DNA during Solid-Phase Synthesis by Oxidative Coupling of Amines to Phosphites |
Contact information | Professor Kurt Gothelf |
