Newly found way to recycle polyurethane, a ubiquitous though complicated plastic material
Polyurethane (PU) is a plastic material used in an infinite number of products. Unfortunately, it is difficult to break down and recycle, which leads to increasing PU waste accumulating every year. However, researchers from Aarhus University have found a way to deconstruct PU, which shows the potential for a circular plastic economy for PU.
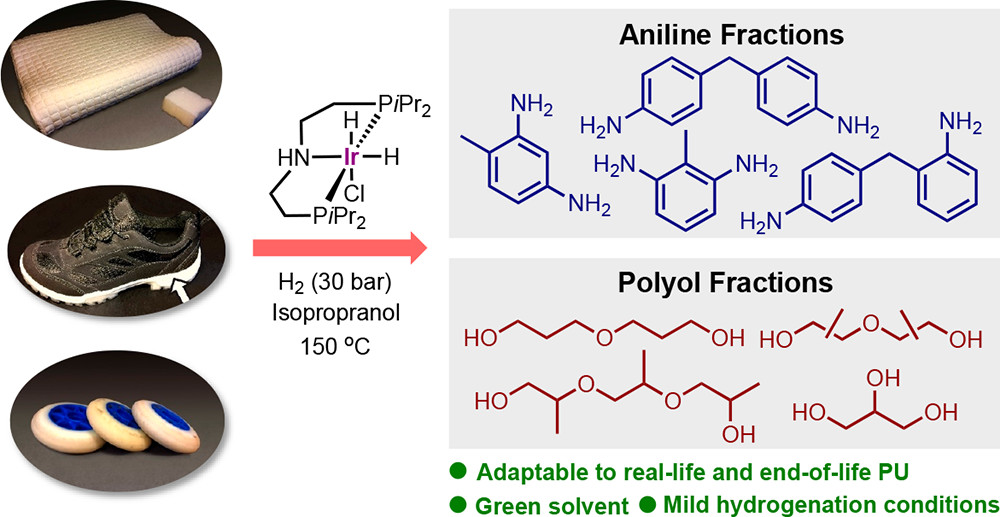
Polyurethane (PU) is a highly valued polymer prepared from diisocyanates and polyols, and it is used in everyday products, such as shoe soles, mattresses, and insulation materials, but also for the construction of sophisticated parts of medical devices, wind turbine blades, aircrafts, and spacecrafts, to name a few.
As PU is most commonly used as a thermoset polymer composed of cross-linked structures, that do not melt when heated. Its recycling is complicated and inefficient, leading to increasing PU waste accumulating every year.
Catalytic hydrogenation represents an atom-efficient means for the deconstruction of polyurethanes, but so far the identification of an efficient catalyst for the disassembly of real-life and end-of-life PU samples has not been demonstrated.
Researchers from Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry have, in collaboration with industry, found a way to deconstruct PU from commercially available products in order for the material to be reused. The results have been published in JACS Au.
In this paper, the researchers reveal that a commercially available catalyst, Ir-iPrMACHO, under 30 bar H2 and 150–180 °C, is a general catalyst for the effective hydrogenation of the four cornerstones of PU: flexible solid, flexible foamed, rigid solid, and rigid foamed, leading to the isolation of aromatic amines and a polyol fraction.
For the first time, a variety of commercial PU materials, including examples of foams, inline skating wheels, shoe soles, and insulation materials, have been deconstructed into the two fractions. Most desirable, the reaction conditions include the use of isopropyl alcohol as a representative of a green solvent. It is speculated that a partial glycolysis at the surface of the PU particles is taking place in this solvent and reaction temperatures in the presence of catalytic amounts of base.
As such a more efficient hydrogenation of the solubilized PU fragments in isopropyl alcohol becomes possible. As the isolated anilines are precursors to the original isocyanate building blocks, and methods for their conversion are well-known, the work reported in this paper provides a realistic indication of a potential circular plastic economy solution for PU.
Read the scientific article in Journal of the American Chemical Society Au (JACS Au):
The research has been carried out by scientists from Interdisciplinary Nanoscience Centre (iNANO) and Department of Chemistry at Aarhus University in collaboration with the RePURpose consortium, incl. Plixxent A/S, T. Dan-Foam ApS, ECCO SKO A/S, H. J. Hansen Genvindingsindustri A/S, Logstor A/S, N. Tinby A/S, and the Danish Technological Institute. Professor Troels Skrydstrup has been in charge of the research team behind the study.
The work was generously supported by the Carlsberg Foundation, Innovation Fund Denmark, and the Danish National Research Foundation.
For further information, please contact:
Professor Troels Skrydstrup
Interdisciplinary Nanoscience Center (iNANO) & Department of Chemistry
Aarhus University
Denmark
Email: ts@chem.au.dk
Assistant Professor Steffan Kvist Kristensen
Interdisciplinary Nanoscience Center (iNANO)
Aarhus University
Denmark
Email: skk@inano.au.dk
