Study Reveals First High-Resolution Structure of Functional Bacterial Amyloid
An international research team led by Professor Daniel Otzen from the Interdisciplinary Nanoscience Center has unveiled the first-ever single-residue resolution structure of a functional bacterial amyloid, based entirely on experimental data.
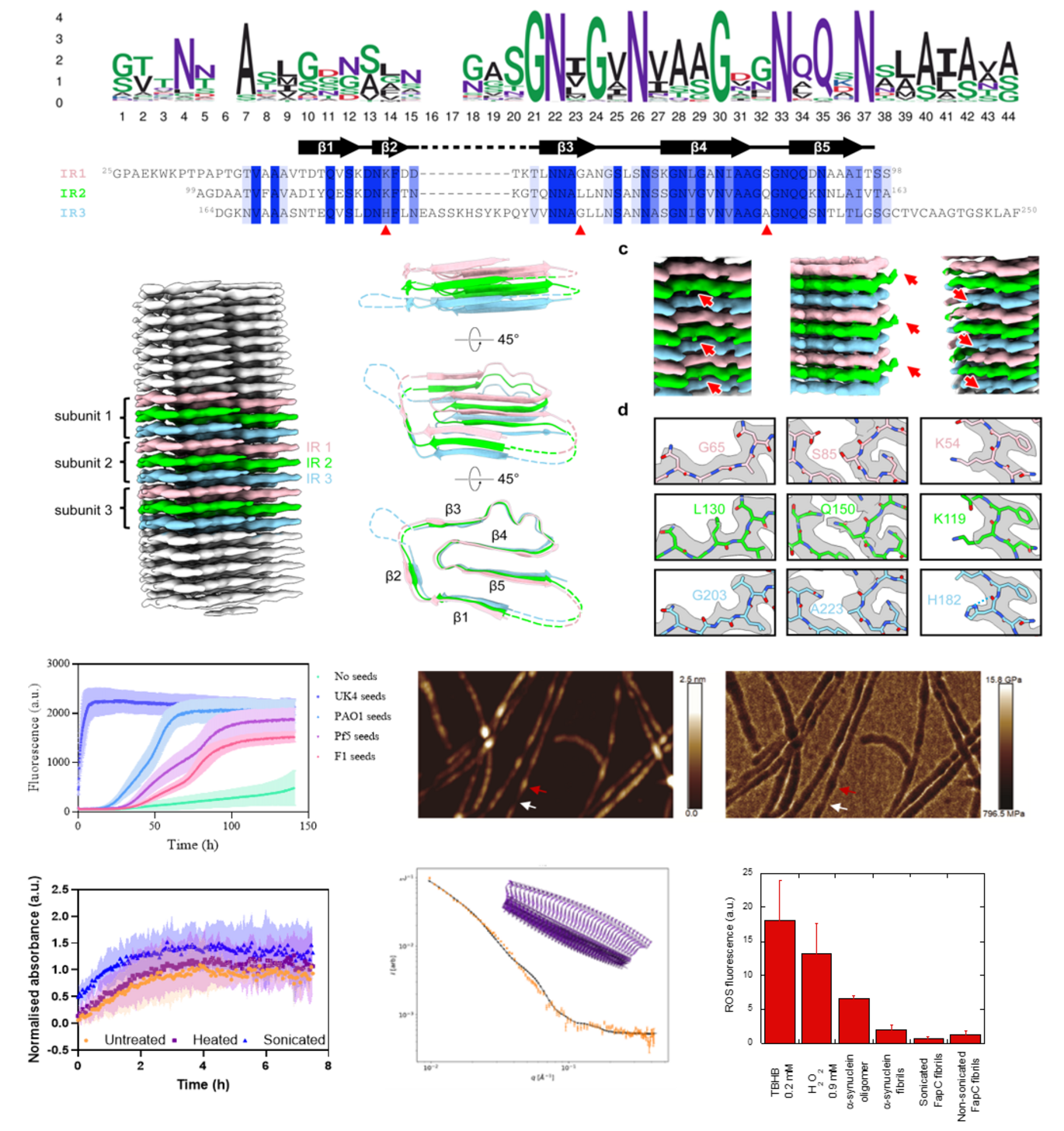
The protein at the center of this breakthrough is FapC, first discovered in 2010 by Otzen in collaboration with his then-PhD student Morten Dueholm and Professor Per Halkjær Nielsen. FapC plays a key role in bacterial biofilms and holds great potential as a bioengineered nanomaterial due to its robustness and modularity.
Read the review published in Molecular Aspects of Medicine here
In the study, published in Advanced Materials (IF 27) this week, the highly interdisciplinary team sheds light on the structure of the FapC protein from Pseudomonas sp. UK4—an essential component of bacterial biofilms. Together with their new insights into the amyloid's nanomechanical and catalytic properties, the study reveals significant potential for bioengineering applications.
Beyond structural insights, the team demonstrated that FapC fibrils exhibit strain-dependent nanomechanical properties and intrinsic catalytic activity—traits that enhance their appeal as a biomaterial. Covariation patterns in the hydrophobic core and additional mutagenesis experiments provide further clues into how disordered monomers transition into highly ordered fibrils.
This achievement reflects the combined efforts of an interdisciplinary research team:
- Cryo-EM structure determination was conducted by Otzen’s former PhD student Huabing Wang and his current PhD student Yanting Jiang in collaboration with Cao Qin at Shanghai Jiao Tong University.
- Sequence alignment was performed by Morten Dueholm and Anders Daugbjerg, helping interpret conserved motifs considering the experimental structure.
- Atomic force microscopy (AFM) and small-angle X-ray scattering (SAXS), conducted by Zhefei Zhang, Marcos Hernández, and Jan Skov Pedersen, explored the nanomechanical properties of flexible linker regions.
- Computational modeling and kinetic analysis using AlphaFold and Amylofit by Samuel Peña-Díaz highlighted how well predictions match reality—showing that AlphaFold3 performs impressively, but with crucial deviations.
- Additional experiments by Janni Nielsen demonstrated that FapC fibrils exhibit no toxicity to mammalian cells, while Peña-Díaz also showed that these fibrils possess promising catalytic properties.
About the study
Study type:
Experimental Molecular Biology
External funding:
H.W. was sup-ported by Joint Project on the Regional High-Incidence Diseases Re-search of Guangxi Natural Science Foundation (Grant No. 2023GXNS-FAA026030), the National Natural Science Foundation of China (Grant No.32460228), and the National Facility for Translational Medicine (Shang-hai). D.E.O. was supported by the Danish Independent Research Foun-dation (Grant No. 3103-00285B), the Novo Nordisk Foundation (GrantNo. NNF22OC007891), and the Lundbeck Foundation (Grant Nos. R276-2018-671 and R453-2024-359)
Conflicts of interest:
The authors declare no conflict of interest
Link to the scientific article:
https://doi.org/10.1002/adma.202505503
Yanting Jiang, Samuel Peña-Díaz, Zhefei Zhang, Anders Ogechi Hostrup Daugberg, Marcos López Hernández, Janni Nielsen, Qiaojie Huang, Shenghan Qin, Morten K. D. Dueholm, Mingdong Dong, Jan Skov Pedersen, Qin Cao, Daniel E. Otzen, and Huabing Wang
Contact information:
Professor Daniel Otzen
Aarhus University
Interdisciplinary Nanoscience Center (iNANO) and the Department of Molecular Biology & Genetics
Email: dao@inano.au.dk
