The ‘Laboratory for Cell Mimicry’ aims to employ bottom up assemble synthetic entities to assembled live-like units that could integrate with their natural counter parts. We work across the length scale, from synthesizing organic compounds as enzyme mimics over the assembly of catalytic nanoparticles as artificial organelles to the engineering of artificial cells and their integration with mammalian cells into bionic tissue.
Artificial organelles are nanosized reactors with intracellular activity, often encapsulated catalysis. In contrast to traditional pharmaceutical, artificial organelles are envisioned to provide a sustained solution for a chronic condition.
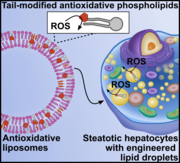
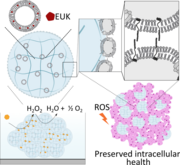
Intracellular reactive oxygen species (ROS) in steatotic cells can cause oxidative stress and damage. To address this, engineered phospholipids were delivered to intracellular lipid droplets in steatotic hepatic cells using the cells' lipid transport mechanisms. Tail-labeled fluorescent lipids formed into liposomes were shown to be transported to lipid droplets in steatotic HepG2 and HHL-5 cells. An antioxidant, a EUK salen–manganese derivative with superoxide dismutase and catalase activity, was covalently attached to a phospholipid tail and formulated as liposomes. Steatotic HepG2 and HHL-5 cells treated with these antioxidant liposomes had lower intracellular ROS levels compared to untreated controls and non-covalently formulated antioxidants. This study demonstrates a new strategy to equip native organelles in mammalian cells with engineered enzyme activity.
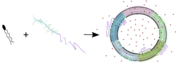
Amphiphilic block copolymers and lipids form hybrid vesicles (HVs), alternatives to liposomes and polymersomes. These copolymers, featuring hydrophobic poly(sitostryl methacrylate) or its copolymers with butyl methacrylate and hydrophilic poly(carboxyethyl acrylate), assemble with soybean L-α-phosphatidylcholine (soyPC) into small HVs. This assembly is confirmed by electron microscopy and small-angle X-ray scattering. Fluorescence resonance energy transfer and Laurdan fluorescence indicate a hybrid membrane structure similar to liposomes but with transmembrane asymmetry. Giant HVs with homogeneous copolymer and soyPC distribution, created via electroformation, show slower lateral diffusion and higher permeability compared to soyPC and sitosterol vesicles. This study advances the understanding of hybrid membranes combining natural and synthetic components.
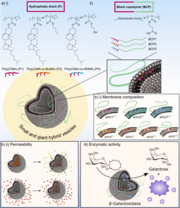
Hybrid vesicles (HVs), formed by combining phospholipids and amphiphilic block copolymers (BCPs), offer a contemporary alternative to liposomes and polymersomes. By altering the chemical composition of the hydrophobic block in the BCPs, specifically using statistical copolymers of cholesteryl methacrylate and either butyl methacrylate (BuMA) or 2-hydroxyethyl methacrylate (HEMA), variations in HV membrane properties were achieved. Membranes with HEMA-containing BCPs exhibited higher permeability, while those with BuMA provided an optimal environment for efficient association with β-galactosidase, demonstrating the significance of the hydrophobic block in controlling HV morphologies and properties.
Artificial cells are larger micron-sized assemblies that aim at structurally and functionally support mammalian cells and tissue.
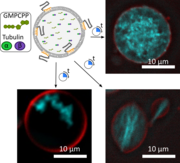
Creating artificial cells with a dynamic cytoskeleton is a key goal in synthetic biology. Here, we demonstrate in situ polymerization of microtubules within giant polymer-lipid hybrid vesicles (GHVs) composed of phospholipids and an amphiphilic block copolymer. The copolymer consists of a hydrophobic poly(cholesteryl methacrylate-co-butyl methacrylate) block and a hydrophilic poly(6-O-methacryloyl-D-galactopyranose) or poly(carboxyethyl acrylate) extension. Microtubule morphology varies with guanosine triphosphate (GTP) or its analog GMPCPP, forming networks, protrusions, or membrane-associated structures. This work advances cytoskeleton mimicry and reveals membrane composition effects on microtubule organization.
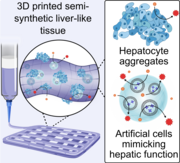
Artificial cells, designed to support mammalian cells, feature minimal liver-like functions using alginate and metalloporphyrins mimicking CYP1A2 enzyme activity. Integrated into 3D bioprinted structures with HepG2 cells, these artificial cells enhance dealkylation activity, monitored by resorufin conversion. The composite ink, combining artificial cells and HepG2 aggregates, sustains CYP1A2-like activity for at least 35 days, demonstrating a promising step in bottom-up synthetic biology for tissue engineering.
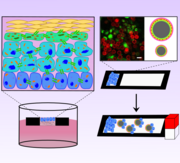
Integrating artificial and mammalian cells into semi-synthetic aggregates remains a challenge in synthetic biology. This study demonstrates the fabrication of cell membrane vesicles (CMVs) from HepG2 cells to coat alginate microgels, creating camouflaged artificial cells (ACs). These ACs facilitate the formation of synthetic and semi-synthetic aggregates. Predator-defendant and liver-like synthetic aggregates show promising potential, while camouflaged ACs enhance integration with HepG2 cells. Encapsulating a ROS-scavenging artificial enzyme in ACs protects HepG2 cells from oxidative stress, improving viability, proliferation, and mitochondrial health. This work advances the fusion of mammalian cells and ACs, with the latter serving as support units.
Self-propelled particles attract a great deal of attention due to the auspicious range of application nanobots can be used for. In a biomedical context, self-propelled swimmers hold promise to autonomously navigate to a desired location in an attempt to counteract cell/tissue defects either by releasing drugs or performing surgical tasks. Self-propelled particles attract a great deal of attention due to the auspicious range of application nanobots can be used for. In a biomedical context, self-propelled swimmers hold promise to autonomously navigate to a desired location in an attempt to counteract cell/tissue defects either by releasing drugs or performing surgical tasks.

This study explores the potential of nano/micromotors for drug delivery by enhancing their collagenase-loading capacity using poly(2-(diethylamino)ethyl methacrylate) polymer brushes. The aim is to investigate the impact of gelatine viscosity, motor size, and morphology on propulsion velocity. Remarkably, both 500 nm and 1 μm motors achieve speeds of up to ∼15 μm s−1 in stiff gelatine-based hydrogels when triggered with calcium. These findings underscore the promise of collagenase-based motors for navigating the extracellular matrix.
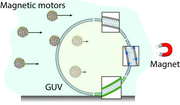
Self-propelled nano/micromotors, crucial for applications in nanomedicine like imaging and drug delivery, were investigated in this study. Magnetically propelled motors of varying sizes and surface chemistries were tested for their ability to traverse lipid membranes in giant unilamellar vesicles (GUVs). Surprisingly, size alone did not dictate membrane-crossing ability; PEGylated motors of 0.5 μm size showed limited interaction compared to their positively charged counterparts of the same size, and membranes with saturated lipids facilitated crossing irrespective of motor size. The study offers valuable insights for future motor designs by considering both ensemble and individual motor behavior in interactions with biological barriers.
A novel in vitro epidermis model using floating paper chips as a scaffold for primary human keratinocytes demonstrates the formation of all four epidermal layers, including the cornified layer, tight junction formation, and organelle alterations during differentiation. This model is employed to assess keratinocyte migration, and magnetic micromotors, assembled on the paper chips, are confirmed to aid cell migration under a static magnetic field, presenting a promising approach for studying skin disease pathologies and treatment evaluations.
Soft actuators are flexible, deformable devices designed to mimic the motion and adaptability of organisms or muscles. They are made from soft, responsive materials, enabling them to bend, stretch, and contract in response to external stimuli. These actuators are used in applications requiring gentle and adaptive movements, such as soft robotics, wearable devices, and biomedical devices.

Hydrogel-based actuators, responsive to external stimuli, are promising for applications like soft robotics. We use 3D printing to create layered rectangles with gelatin methacryloyl (GelMA) and GelMA-poly(N-isopropylacrylamide) (PNIPAM) inks, evaluating their bending capabilities in a buffer. Gold nanostars act as photothermal elements, enabling repeated bending of a 3D-printed fish tail under near-infrared light, demonstrating the potential of combining 3D printing, responsive hydrogels, and photothermal elements for soft robotics.