Currently we investigate ZSM-5 zeolites, the archetype MTH catalyst (methanol-to-hydrocarbon). ZSM-5 exhibits the MFI structure consisting of a 3D network of different ring channels and 24 crystallographically distinct tetrahedral sites which provide many different possible ways of Al substitution into the silicate framework. Solid-state NMR spectroscopy helps us to detect and understand in which way the Al atoms are distributed over the distinct tetrahedral sites and the effect of the distribution of Al on the catalytic behavior. We investigate how the distribution can be affected by different synthesis methods and how the Al sites alter during reactivation treatments.
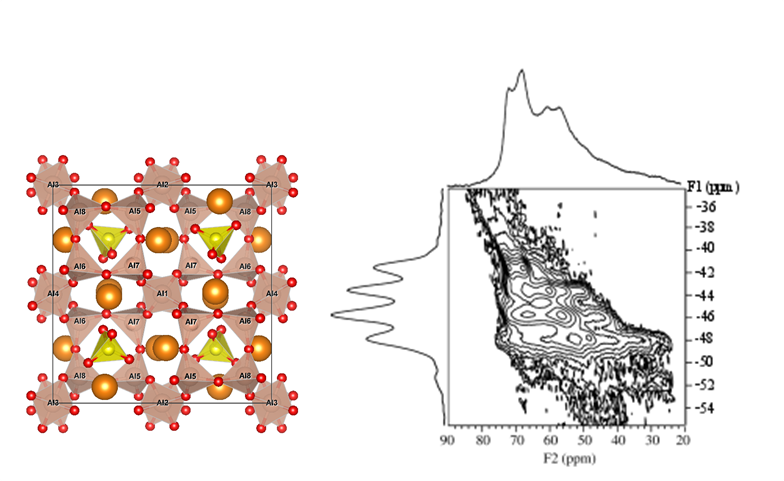
Calcium sulfo-aluminate (CSA) cement is an alternative to Portland cement which attracts significant attention due to its lower embodied CO2. The main component in CSA cement is ye’elimite (Ca4Al6O12SO4), which exhibits a structure with 8 different Al sites all tetrahedrally coordinated to oxygen (left). In order to identify the distinct Al sites and determine their characteristic quadrupole coupling parameters 27Al NMR is utilized. The 27Al MQMAS spectrum at the right shows how a higher resolution of sites is achieved by eliminating the anisotropic part of the quadrupole coupling (vertical projection) compared to the MAS spectrum (horizontal projection).
Concrete production has a significant, direct impact on global CO2 emissions mostly due to the decarbonation of calcium carbonate (chalk) during cement production and the vast amounts of concrete used to provide and maintain infrastructures for human use. The partial replacement of traditional cements by supplementary cementitious materials (SCM’s), such as fly ash, slag and calcined clay, reduces the CO2 footprint of cement production, but also alters the chemical composition of the main hydration product responsible for the binding capability of these materials: the calcium-silicate-hydrate (C-S-H) phase. Generally, research and model development for the microstructure of hydrated cements focus on C-S-H phases with high Ca/Si ratios which are typical for traditional Portland cements, whereas Portland cement – SCM blends often have much lower Ca/Si ratios.
 |
|---|
Figure: a) tobermorite-like C-S-H structure of CaO2 layers (orange) with chains of SiO4 tetrahedra (red triangles) in a dreierketten arrangement, and sketches of possible microstructural models indicating different environments for water (b and c) in combination with a schematic 1H NMR correlation spectrum of T1 and T2 relaxation times (d). |
The aim of this project is to characterize the microstructural development of cements with different Ca/Si ratios with low-field NMR and other techniques and thereby explore relationships between nano-pores and mechanical properties of cementitious materials. The working hypothesis is that the quantity and distribution of water molecules between the hydrate phases in hardened cement increases with decreasing Ca/Si ratios of the cements (as obtained by the use of SCM´s). A larger amount of water, finely distributed in the microstructure, may explain the improved space filling, the reduced permeability, and the higher compressive strengths of cements blended with SCM’s.
NMR techniques are very valuable to investigate the X-ray amorphous C-S-H phases of variable composition. High-resolution, high-magnetic field solid-state NMR work in our group has delivered many important contributions to the understanding and verification of structures of C-S-H phases on the atomic scale. In the present project we want to add information on the nano- to micrometer scale by quantifying the so called gel-water surrounding the solids in hardened cements by low-magnetic field 1H NMR that probes T1 and T2 relaxation times.
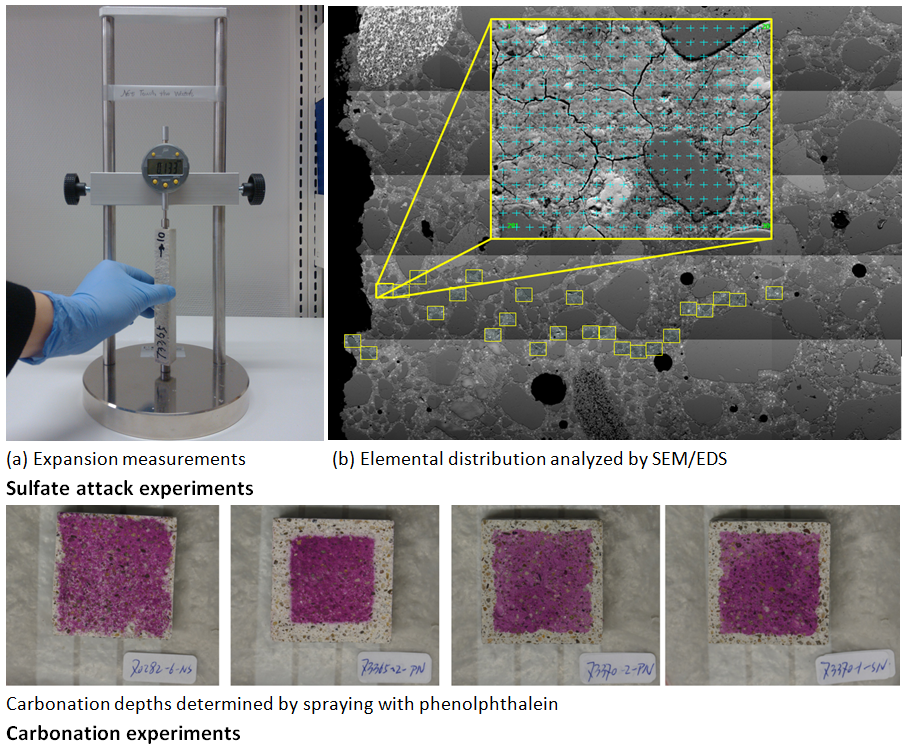
New sustainable cements are being developed in our laboratory by replacing a significant fraction of the CO2-intensive Portland cement with alternative materials such as calcined clays (e.g. metakaolin and montmorillonite) and limestone. The combined use of calcined clays and limestone allows replacements of Portland cement as high as 35 wt.% without sacrificing compressive strengths. The durability performance of these new blended cements is being investigated by studying their interactions with sulphate, chloride and carbonate ions. Multi-disciplinary approaches are used to identify and quantify phase changes (e.g. XRD and TGA) in these systems and to characterize their microstructures (e.g. MIP, SEM, AC impedance). In addition to the experimental evaluations, thermodynamic modelling is used to predict the changes in phase assemblages and porosities. Comparison of the experimental data and thermodynamic calculations allow us to explore the involved reactions and provide fundamental knowledge for both qualitative and quantitative analysis. The results contribute to a better understanding of several deterioration mechanisms for Portland cement blends, which is important for exploring the key factors that control the durability performance of new Portland cement – calcined clay – limestone blends.