Thermal Analysis
Thermal analysis generally covers three different experimental techniques:
The Department of Chemistry has equipment to carry out both a TGA and DTA in the range of 100 - 1500 K, and DSC measurements in the range of 100 - 1000 K. During all experiments a selection of crucibles are available (e.g., platinum, gold, aluminum, quartz) and the measurements can be done in a flow of different gases.
The basic principle in TGA is to measure the mass of a sample as a function of temperature. In principle, this simple measurement is an important and powerful tool in solid state chemistry and materials science. The method, e.g., can be used to determine water of crystallisation, follow degradation of materials, reaction kinetics, study oxidation and reduction, and teach the principles of stoichiometry, formulae and analysis.
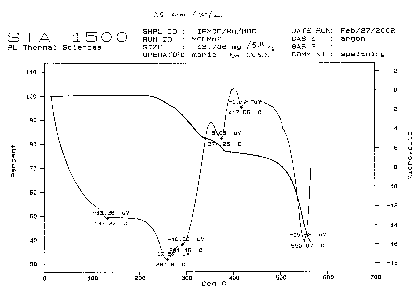
The TGA and DTA curves of a new magnetic material with a nanoporous framework structure
Many thermal changes in materials (e.g. phase transitions) do not involve a change of mass. In DTA one instead measures the temperature difference between an inert reference and the sample as a function of temperature. When the sample undergoes a physical or chemical change the temperature increase differs between the inert reference and the sample, and a peak or a dip is detected in the DTA signal. The technique is routinely applied in a wide range of studies such as identification, quantitative composition analysis, phase diagrams, hydration-dehydration, thermal stability, polymerisation, purity, and reactivity.
DSC uses the same basic principles as DTA with an inert reference material and a sample. However, in DSC the temperature of the sample is kept identical to the reference material during the heating. Thus, if a change in temperature is detected between the sample and the reference, the input power to the sample is changed to equilibrate the temperature. In this way one can quantitatively determine the heat input(output) to(from) the sample during a phase transition.