It is expected that engineered nanomaterials will be increasingly and widely used in e.g. biomedical applications and in consumer products. Whereas there is a clear benefit using nano-formulations as drug delivery systems, there are health concerns especially in relation to exposure from nanoparticles present in consumer products e.g. cosmetics. As nanoparticles can have different physicochemical properties from their bulk chemical forms, it has been suggested that a new paradigm for risk assessment must be developed, e.g. different exposure metrics. Another concern is if the current testing methods validated by OECD are appropriate to test the safety of nanoparticles released from engineered nanotechnology materials.
The objective of this project is to use an in vivo/in vitro parallelogram approach to identify targets of nanoparticle toxicity, and to investigate the molecular mechanism of the toxicity with focus on genotoxicity and inflammatory responses. The focus of these studies will be on routes relevant for human exposure i.e. inhalation and oral intake.
Linking in vivo and in vitro toxicological data will shed light on the potential application of cell culture models to assess the risk of nanoparticles without detailed epidemiological studies that are usually difficult in the case of new technology materials. Furthermore, comparative analyses of the effects in tissues of the same origin from mouse and human may indicate whether there is a similarity in the sensitivity and mechanism of toxicity. In the in vitro models, the questions on the appropriate dose metrics will be addressed, an emerging issue that is of high importance for the risk characterisation of nanoparticles.
In order to validate the parallelogram testing approach, 4 different classes of NPs have been selected – CeO2, TiO2, Ag and high-aspect-ratio (HAR) nanotubes.
A range of analytical techniques are employed to investigate the physicochemical properties of nanoparticles (e.g. size, surface area, chemical and molecular composition and surface interactions) before and after exposure to biological fluids. The analysis makes use of information rich spectroscopic tools such as Time-of-Flight Secondary Ion Mass Spectroscopy (ToF-SIMS), X-ray Photoelectron Spectroscopy (XPS), X-ray Diffraction (XRD), Small Area Electron Diffraction (SAED). A combination of in situ (in fluids) and ex situ nanoimaging and spectroscopic tools are used to evaluate the size and surface chemistry of the nanoparticles e.g. Dynamic Light Scattering (DLS), Small-Angle X-ray Scattering (SAXS), Transmission Electron Microscopy (TEM) and Atomic Force Microscopy (AFM) and optical spectroscopies (for metal nanoparticles such as Ag). The Inductively-Coupled Plasma Mass Spectrometry (ICP-MS) provides useful information for chemical and molecular composition.
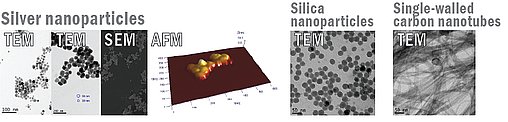
One of the major issues in the area of nanotoxicology is the characterisation of the nanoparticles in biological fluids, as the intrinsic physicochemical properties can change by the incorporation of biological materials (e.g. macromolecules), a process that is likely to modify the uptake and the toxicity of the nanoparticles. The interaction of biological molecules with nanoparticles is studied in model experiments and by identification of macromolecules (through proteomic approaches) recovered from nanoparticles exposed to biological fluids. The detailed characterisation and evaluation of the effects of modifications occurring to the nanoparticles en route to cells and during entry into tissue will provide new evaluation criteria for specific nanoparticle hazard and exposure in terms of risk assessment, for example the relative potential for a specific nanoparticles to be modified in particular ways.
The relevant routes of exposure for nanoparticles are likely to be the lung via inhalation, the gastrointestinal tract or skin, thus the focus is on inhalation and oral uptake. The distribution of the nanoparticles following inhalation and oral uptake are assessed in mouse using both physical/chemical methods and biomarkers. The primary cells such as macrophages and T cells are isolated and collected for the analysis of inflammatory responses following exposure to nanoparticles. Gene expression assays for toxicity pathways and induction of DNA damages are analysed in the animal tissues following exposure, and relevant genes will be identified.
The in vitro toxicological studies are carried out in pairs of human and murine cells. Lung cells A549 (human lung carcinoma), BEAS-2B (SV40-human bronchial epithelial cells), mouse LA-4 (epithelial lung adenoma) and Human colon Caco-2 (adenocarcinoma), mouse monocyte/macrophage (RAW 264.7) and human monocytes THP-1, and human brain HCN-1 (neuronal) and mouse SW40 (neuronal) cells.
The dose-response relationship of biomarkers are investigated as a function of different exposure metrics e.g. surface area, number and mass. The determined IC10 and IC50 concentrations are used in all other toxicological studies in order to cover responses at low and intermediate/high exposure.
Oxidative stress is considered central to the toxicological mechanism of action for nanoparticles. Damage to DNA is primarily induced by reactive oxygen and nitrogen species. Thus, the intracellular production of ROS is assessed by the fluorogenic dye H2DCF-DA, and oxidative stress are estimated by the GSH/GSSG ratio as well as malondialdehyde (MDA) levels. Damage to the cells and apoptotic cells are measured using the annexin V/propidium iodide flow cytometric assay.
Key genes in the toxicological pathways are identified using PCR-based assays. One of the proposed toxicological mechanisms by which nanoparticles exert their proinflammatory effects are through the generation of oxidative stress. Cellular responses to oxidative stress include activation of antioxidant defence mechanisms.
Inflammation is a complex biological process involving different cell types, and thus cannot be measured in simple in vitro assays. In this project we focus on markers of proinflammatory signalling e.g. cytokines and chemokines that play specific roles in promoting or controlling inflammation. A PCR-based assay is used to quantify TNFα, IL1α, IL1β, IL6 and IL8. These markers have been selected based on sporadic studies reporting that the expression levels are altered by ultra-fine particles, including diesel exhaust particles (DEP) and some nanoparticles.
The concern for potential carcinogenicity of nanoparticles is based upon the increased cancer risk following exposure for asbestos and DEP and recent studies showing the induction of mesothelium by carbon nanotubes in p53 deficient mice at high doses. In addition, some nanoparticles have demonstrated genotoxic activity in different in vivo models using transgenic animals and in cell cultures. Development of cancer can operationally be divided into genotoxic and non-genotoxic events.
- Genotoxicity
To investigate the genotoxicity of nanoparticles, the potential target tissues are studied using international standard OECD protocols for analysing chromosomal mutations (chromosomal aberrations), formation of DNA strand breaks (the comet assay) and induction of aneuploidy, and using the P32 post-labelling techniques for analysing unspecific bulky DNA adducts. Of the four events named above, chromosomal mutations and DNA strand breaks are believed to be due to direct DNA damage, whereas aneuploidy could be induced by the interference of nanoparticles with the spindle-apparatus. Preliminary studies have shown that nanoparticles induce bulky DNA adducts in cultured human cells in a dose-dependent manner and the level depends on the type and size of the nanoparticles. These adducts may be formed as a secondary effect of oxidative stress induced by nanoparticles, e.g. lipidperoxidation products. The relevance of this marker to assess risk has been demonstrated in epidemiological studies where bulky DNA adduct level is a risk indicator for lung cancer.
- Non-genotoxic (epigenetic) events
Different mechanisms for non-genotoxic carcinogens have been proposed, one of these is a change in methylation pattern that may result in altered gene expression. Hypermethylation of the CpG island in region of certain tumor repressor genes are known to be associated with many human tumors. Both global methylation level and gene specific methylation are investigated. We have developed several methods to study epigenetic mechanisms on the regulation of specific gene expressions e.g. transferrin receptor methylation specific PCR (MSP) technique, which is a bisulfite conversion based PCR technique. Furthermore, bisulfite sequencing can quantify the methylation status of cytosines located in specific DNA regions. A chemiluminescent electrophoretic mobility shift assays (EMSA) to study protein-DNA interactions involved in DNA epigenetic regulation is also employed.
It is anticipated that the information obtained from this project mostly will be of interest to the regulatory and academic communities as a contribution to the ongoing debate on the evaluation of the safety of materials/products that contain nanoparticles. The focus of this approach is on the 3R-principles.