The purpose of this project is to explore the encapsulation potential of a new generic class of protein-lipid complexes, which we call liprotides.
We define liprotides as complexes formed between a wide range of different proteins and free fatty acids [1]. Liprotides have same generic structure, named core-shell, with a micelle-like core of fatty acid and a shell of partially denatured proteins, Figure 1.
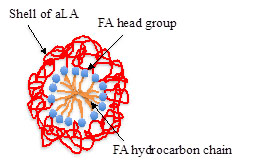
The primary function of the protein shell is assumed to be to enhance the solubility of the fatty acid. The ability to enhance the solubility of fatty acids gives liprotides the potential to function as a carrier of hydrophobic molecules in a hydrophilic environment.
Another function of the protein shell is to carry and deliver the fatty acid to a cell or other hydrophobic surfaces. Liprotides are toxic for cells and the fatty acid attributes to the toxic effect of the complex. The cytotoxicity of liprotides allows these complexes to be used as a carrier of hydrophobic drug molecules.
In this PhD project the focus is: incorporation of hydrophobic molecules in liprotides for applications within the food, cosmetic and pharmaceutical industries. Before liprotides can be used for different applications, it is important to investigate the following topics:
PhD student Henriette Søster Frislev supervised by Daniel Otzen. Work initiated October 2014.
Co-worker: M.Sc. Jannik Nedergaard Pedersen.
The project will be carried out at the laboratory of Daniel Otzen.
We collaborate with Professor Jan Skov Pedersen using Small Angle X-Ray Scattering to investigate liprotide structures and with Assoc. Professor Rikke Louise Meyer on the impact of liprotides on fighting bacterial biofilms.
[1] J.D. Kaspersen, J.N. Pedersen, J.G. Hansted, S.B. Nielsen, S. Sakthivel, K. Wilhelm, E.L. Knyazeva, E.A. Permyakov, E.A. Permyakov, C.L.P. Oliveira, L. Morozova-Roche, D.E. Otzen, J.S. Pedersen, (joint corresponding authors) Generic structures of liprotides, complexes between partially denatured proteins and oleic acid: a fatty acid core with a shell of disordered proteins, ChemBioChem, In press (2014).